Reducing Agents
Vue d'ensemble
Source: Vy M. Dong and Daniel Kim, Department of Chemistry, University of California, Irvine, CA
Controlling the reactivity and selectivity during the synthesis of a molecule is very important criteria for chemists. This has led to the development of many reagents that allow chemists to pick and choose reagents suitable for a given task. Quite often, a balance between reactivity and selectivity needs to be achieved. This experiment will use IR spectroscopy to monitor the reaction and to understand the reactivity of carbonyl compounds as well as the reactivity of hydride-reducing reagents.
Procédure
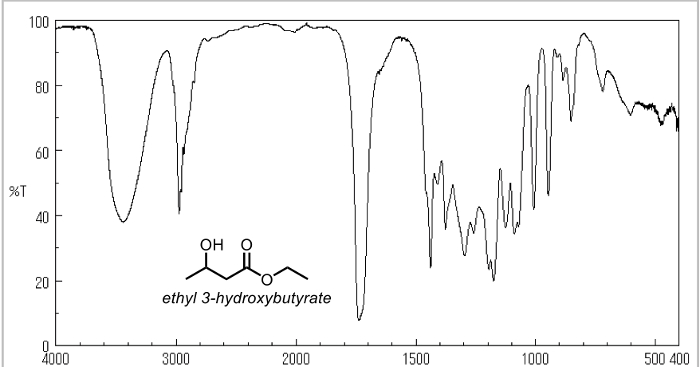
1. Measuring Properties of Ethyl Acetoacetate
- Take an IR of the starting material (ethyl acetoacetate).
- Take a TLC using 40% ethyl acetate in 60% hexanes.
2. Reduction of Ethyl Acetoacetate with Sodium Borohydride
- Add 1 mmol of ethyl acetoacetate to a round-bottom flask.
- Add 5 mL of ethanol and swirl to mix completely.
- Lower the beaker into an ice-water bath.
- Weigh 1 mmol of sodium borohydride and slowly add to the stirred
Résultats
Applications et Résumé
The trends of carbonyl reactivity and hydride donor ability have been reviewed and demonstrated. The visual reactiveness of the two commonly used reagents is apparent and can be appreciated.
The understanding of the reactivity of reagents and functional groups is of high importance when developing new methods for reductions or any other kind of reaction. Controlling the selectivity and reactivity of any reaction is an important factor to consider when deciding on the reagents used for a chemic
Passer à...
Vidéos de cette collection:

Now Playing
Reducing Agents
Organic Chemistry II
42.8K Vues

Cleaning Glassware
Organic Chemistry II
123.1K Vues

Nucleophilic Substitution
Organic Chemistry II
99.2K Vues

Grignard Reaction
Organic Chemistry II
148.8K Vues

n-Butyllithium Titration
Organic Chemistry II
47.6K Vues

Dean-Stark Trap
Organic Chemistry II
99.8K Vues

Ozonolysis of Alkenes
Organic Chemistry II
66.8K Vues

Organocatalysis
Organic Chemistry II
16.6K Vues

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.2K Vues

Solid Phase Synthesis
Organic Chemistry II
40.8K Vues

Hydrogenation
Organic Chemistry II
49.3K Vues

Polymerization
Organic Chemistry II
93.7K Vues

Melting Point
Organic Chemistry II
149.6K Vues

Infrared Spectroscopy
Organic Chemistry II
214.0K Vues

Polarimeter
Organic Chemistry II
99.8K Vues