Freezing-Point Depression to Determine an Unknown Compound
Source: Laboratory of Lynne O' Connell — Boston College
When a solid compound is dissolved in a solvent, the freezing point of the resulting solution is lower than that of the pure solvent. This phenomenon is known as freezing-point depression, and the change in temperature is directly related to the molecular weight of the solute. This experiment is designed to find the identity of an unknown compound by using the phenomenon of freezing-point depression to determine its molecular weight. The compound will be dissolved in cyclohexane, and the freezing point of this solution, as well as that of pure cyclohexane, will be measured. The difference between these two temperatures allows for the calculation of the molecular weight of the unknown substance.
A temperature probe interfaced to a computer is used to acquire the temperature readings in this experiment. The temperature probe has an uncertainty of ±0.1 °C.
1. Setting the Parameters in the Software
- Set the length of the experiment to 800 s.
- Set the sampling rate to 1 sample per second.
- Set the upper limit for the temperature range to 40 °C and the lower limit to 0 °C.
2. Measuring the Freezing Point of Cyclohexane
The mass of cyclohexane that was dispensed can be calculated. The density of cyclohexane is 0.779 g/mL.
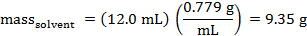
The values for Tf° and Tf can be determined from the plots.
The molar mass, and thus the molecular weight, of the
Perhaps the most visible application of the phenomenon of freezing-point depression occurs during the winter months, when roads and sidewalks become icy, and salt is used to treat the slippery surfaces. When the salt mixes with the ice, the freezing point of the water is depressed so the ice melts at a lower temperature. Because the degree of the freezing point depression is dependent on the number of particles in solution, salts that release three ions per formula unit, such as calcium chloride (CaCl2), are o
Vai a...
Video da questa raccolta:

Now Playing
Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.3K Visualizzazioni

Common Lab Glassware and Uses
General Chemistry
652.7K Visualizzazioni

Solutions and Concentrations
General Chemistry
272.6K Visualizzazioni

Determining the Density of a Solid and Liquid
General Chemistry
554.3K Visualizzazioni

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
382.9K Visualizzazioni

Determining the Empirical Formula
General Chemistry
179.1K Visualizzazioni

Determining the Solubility Rules of Ionic Compounds
General Chemistry
140.9K Visualizzazioni

Using a pH Meter
General Chemistry
344.1K Visualizzazioni

Introduction to Titration
General Chemistry
423.0K Visualizzazioni

Ideal Gas Law
General Chemistry
77.8K Visualizzazioni

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.2K Visualizzazioni

Le Châtelier's Principle
General Chemistry
263.7K Visualizzazioni

Determining Rate Laws and the Order of Reaction
General Chemistry
195.6K Visualizzazioni

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Visualizzazioni

Coordination Chemistry Complexes
General Chemistry
91.1K Visualizzazioni