Hydrogenation
Source: Vy M. Dong and Zhiwei Chen, Department of Chemistry, University of California, Irvine, CA
This experiment will demonstrate the hydrogenation of chalcone as an example of an alkene hydrogenation reaction (Figure 1). In this experiment, palladium on carbon (Pd/C) will be used as a heterogeneous catalyst for the process. A balloon will be used to supply the hydrogen atmosphere.
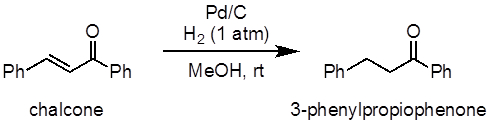
Figure 1: Diagram showing the hydrogenation of chalcone to 3-phenylpropiophenone.
- Add 210 mg of chalcone, 12 mg of 5% Pd/C, and 8 mL of MeOH to a 25 mL round-bottom flask equipped with a magnetic stir bar.
- Seal the round-bottom flask with a rubber septum and start stirring the reaction mixture.
- Obtain a balloon of hydrogen from the hydrogen gas cylinder and set aside.
- With a needle, apply a vacuum to the reaction mixture until bubbling is observed.
- Stop the vacuum and insert the hydrogen balloon.
- After 30 s, remove the hydrogen balloon.
- Repeat steps 4-6, t
3-Phenylpropiophenone was obtained as a white solid (150 mg, 71% yield); m.p. 65-70 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 7.2 Hz, 2H), 7.59 (t, J = 7.2 Hz, 1H), 7.49 (t, J = 7.6 Hz, 2H), 7.37-7.26 (m, 5H), 3.35 (t, J = 7.2 Hz, 2H), 3.12 (t, J = 7.6 Hz, 2H)
In this experiment, we have demonstrated a catalytic hydrogenation reaction of an alkene. Chalcone was hydrogenated to form 3-phenylpropiophenone.
Hydrogenation is an exothermic reaction (releases heat) because the product alkane is more stable than the reactant alkene. The amount if heat released from the reaction can serve as an indicator of the stability of the alkene. In the food industry, hydrogenation is used for processing vegetable oils, which are triglycerides bearing multiple alkene
スキップ先...
このコレクションのビデオ:

Now Playing
Hydrogenation
Organic Chemistry II
49.1K 閲覧数

Cleaning Glassware
Organic Chemistry II
123.1K 閲覧数

Nucleophilic Substitution
Organic Chemistry II
99.1K 閲覧数

Reducing Agents
Organic Chemistry II
42.7K 閲覧数

Grignard Reaction
Organic Chemistry II
148.7K 閲覧数

n-Butyllithium Titration
Organic Chemistry II
47.6K 閲覧数

Dean-Stark Trap
Organic Chemistry II
99.7K 閲覧数

Ozonolysis of Alkenes
Organic Chemistry II
66.7K 閲覧数

Organocatalysis
Organic Chemistry II
16.6K 閲覧数

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.2K 閲覧数

Solid Phase Synthesis
Organic Chemistry II
40.8K 閲覧数

Polymerization
Organic Chemistry II
93.6K 閲覧数

Melting Point
Organic Chemistry II
149.6K 閲覧数

Infrared Spectroscopy
Organic Chemistry II
213.8K 閲覧数

Polarimeter
Organic Chemistry II
99.8K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved