A subscription to JoVE is required to view this content. Sign in or start your free trial.
Closed-Loop Neurostimulation for Biomarker-Driven, Personalized Treatment of Major Depressive Disorder
In This Article
Summary
Deep brain stimulation triggered by a patient-specific neural biomarker of a high-symptom state may better control symptoms of major depressive disorder compared to continuous, open-loop stimulation. This protocol provides a workflow for identifying a patient-specific neural biomarker and controlling the delivery of therapeutic stimulation based on the identified biomarker.
Abstract
Deep brain stimulation involves the administration of electrical stimulation to targeted brain regions for therapeutic benefit. In the context of major depressive disorder (MDD), most studies to date have administered continuous or open-loop stimulation with promising but mixed results. One factor contributing to these mixed results may stem from when the stimulation is applied. Stimulation administration specific to high-symptom states in a personalized and responsive manner may be more effective at reducing symptoms compared to continuous stimulation and may avoid diminished therapeutic effects related to habituation. Additionally, a lower total duration of stimulation per day is advantageous for reducing device energy consumption. This protocol describes an experimental workflow using a chronically implanted neurostimulation device to achieve closed-loop stimulation for individuals with treatment-refractory MDD. This paradigm hinges on determining a patient-specific neural biomarker that is related to states of high symptoms and programming the device detectors, such that stimulation is triggered by this read-out of symptom state. The described procedures include how to obtain neural recordings concurrent with patient symptom reports, how to use these data in a state-space model approach to differentiate low- and high-symptom states and corresponding neural features, and how to subsequently program and tune the device to deliver closed-loop stimulation therapy.
Introduction
Major depressive disorder (MDD) is a neuropsychiatric disease characterized by network-level aberrant activity and connectivity1. The disease manifests a variety of symptoms that vary across individuals, fluctuate over time, and may stem from different neural circuits2,3. Approximately 30% of individuals with MDD are refractory to standard-of-care treatments4, highlighting a need for new approaches.
Deep brain stimulation (DBS) is a form of neuromodulation in which electrical current is delivered to targeted areas of the brain with the goal of modulating the activity. DBS for the treatment of MDD has been very successful in some applications5,6, but has also failed to replicate in larger studies7,8. All of the cited studies employed open-loop stimulation9, in which the delivery of putative therapeutic stimulation was continuous with fixed parameters. In contrast, closed-loop stimulation delivers stimulation based on a programmed biomarker or neural activity pattern associated with the symptom state10. There are two main implementations of closed-loop stimulation: responsive stimulation and adaptive stimulation11. Responsive stimulation delivers bursts of stimulation with constant parameters (e.g., frequency, amplitude, pulse width) when the programmed criteria are met. With adaptive stimulation, stimulation parameters dynamically change as a function of the measured biomarker, according to the algorithm, which may have multiple fix points or automated continuous adjustment. Stimulation can be continuous or intermittent with adaptive stimulation. Adaptive stimulation has shown superior efficacy to open-loop stimulation in controlling symptoms of Parkinson's disease12. Responsive neurostimulation for epilepsy13 is Food and Drug Administration (FDA)-approved, while early investigations of responsive stimulation for MDD14 and adaptive stimulation for Tourette syndrome15 and essential tremor16 also show therapeutic benefit.
To implement closed-loop stimulation, a physiological signal must be selected and tracked to inform when stimulation should be delivered. This feedback is the key difference between open-loop and closed-loop stimulation and is realized by selecting a biomarker. This protocol provides a procedure for determining a personalized biomarker according to the constellation of symptoms experienced by a given individual. Future meta-analyses across patients will reveal if there are common biomarkers across individuals or if the heterogeneous presentation of MDD symptoms and the underlying circuitry necessitates a personalized approach17,18. Using DBS devices capable of both sensing neural activity and delivering electrical stimulation allows for both the discovery of this biomarker and the subsequent implementation of closed-loop neuromodulation. This approach presupposes a close temporal relationship between neural activity and specific symptom states and may not be applicable for all indications or symptoms.
While indications such as Parkinson's disease and essential tremor have symptoms that can be measured using peripheral sensors (e.g., tremor, rigidity), symptoms of MDD are typically reported by the patient or assessed by a clinician using standardized questions and observation. In the context of amassing sufficient data to calculate a personalized biomarker, clinician assessments are not practical, and thus patient reports of symptoms through rating scales are used. Such scales include visual analog scales of depression (VAS-D), anxiety (VAS-A), and energy (VAS-E)19, and the six-question form of the Hamilton Depression Rating Scale (HAMD-6)20. Concurrent recordings of neural activity and completion of these self-report symptom ratings provide a paired dataset that can be used to look at relationships between spectral features of the neural signal related to or predictive of high-symptom states.
Computational approaches, such as state-space modeling, can be used to uncover relationships between symptom states and neural features. Graph theoretic methods are attractive for characterizing a state-space21 because they enable the discovery of states over different timescales by explicitly modeling the temporal proximity between measurements22. A symptom state-space model identifies periods of time in which there is a common phenotype of the patient's symptoms and may pinpoint symptom sub-states in which the ratings on specific dimensions of the patient's depression differ based on the environment or context. A closed-loop approach relies on the detection of symptom states based on underlying brain activity. Machine learning classification is a final step that helps identify a combination of statistical features derived from brain activity signals that best distinguishes two or more symptom states14. This two-stage approach explains the variability in a patient's symptoms over time and links systematic patterns of symptom variation to brain activity.
The present protocol utilizes the NeuroPace Responsive Neurostimulation System (RNS)13,23. Procedures to determine the optimal stimulation site(s) and parameters are outside the scope of this protocol. However, the stimulation capabilities of a given device are important to consider when designing closed-loop neurostimulation. For the device used in this protocol, stimulation is current-controlled and delivered between the anode(s) and cathode(s). One or more electrode contacts or the Can (implantable neurostimulator [INS]) can be selected as the anode(s) or cathode(s). Stimulation frequency (1-333.3 Hz), amplitude (0-12 mA), pulse width (40-1000 µs per phase), and duration (10-5000 ms, per stim) are all pre-programmed. The prior parameters can be set independently for up to five stimulation therapies; these therapies are delivered sequentially if the detection criteria continue to be met. It is not possible to deliver multiple stimulation waveforms simultaneously (e.g., one cannot deliver two different frequencies of stimulation concurrently). The stimulation waveform is a symmetric biphasic rectangular wave and cannot be changed.
Protocol
This protocol has been reviewed and approved by the University of California, San Francisco Institutional Review Board.
1. Device setup for patient at-home recordings
- Work with a representative from the device company to set four configured channels for acquisition, two from each implanted lead.
NOTE: Each channel records a bipolar recording. Configured channels may use adjacent (e.g., 1-2, 3-4) or interleaved (e.g., 1-3, 2-4) electrode contacts. When leads with 10 mm spacing are implanted, adjacent contacts are typically used. When leads with 3.5 mm spacing are implanted, either adjacent or interleaved contacts are used. This is determined by examining the reconstruction of electrode implant location relative to anatomical targets and examining the amplitude of signals. If adjacent contacts produce low amplitude signals, interleaved contacts are preferable. Each contact can only be used in the montage once. - Ensure the patient has fully recovered from surgery following the implantation of the device with cortical and/or depth leads (see24 for additional information on implantation technique).
- Connect the telemetry wand to the programmer (clinician tablet) and have the patient hold the wand over their INS or attach it to a tethering hat (custom-made, not part of the device system; see Figure 1).
- Using the programmer, log on to the patient data management system (PDMS; https://pdms.neuropace.com/login.php), navigate to the correct patient, and select Programming and then Change ECoG Capture. Set the Capture Window to the maximum of 240 s for the four configured channels using the dropdown selection.
NOTE: The device sampling rate is 250 Hz. - On the same settings page, set Reserve Space for magnet reservations to two and to zero for all other trigger types using the dropdown selections. This allows for prioritized saving of two recordings triggered by magnet swipes.
NOTE: Scheduled storage can also be enabled, for automatic and non-prioritized saving of neural recordings at set times of day. These recordings will not be used for determining a biomarker but may be useful for other purposes. - Synchronize the newly programmed settings with the patient's INS by selecting the Review & Program button, confirming changes shown in the presented table, and selecting the Confirm Programming button.
NOTE: Detection and Stimulation should both be set to Disabled.
2. Symptom collection during patient at-home recordings
- Prepare the web-based survey for the patient symptom report (e.g. REDCap25), including sliders for VAS-D, VAS-A, VAS-E, and selection responses for each question of the HAMD-6. Ensure the time of survey start and completion is logged.
- Provide the patient with the unique URL generated by REDCap to access the symptom surveys either via text message or email
3. Procedure for concurrent at-home symptom reports and neural recordings
- Instruct the patient to set up the equipment, including a remote monitor (patient laptop) and wand, magnet, and device for completing survey (e.g. smartphone, tablet, or computer) (Figure 1). Steps 3.2-3.8 are performed by the patient.
NOTE: Most patients quickly learn this procedure. In-person training sessions while the patient is still in the hospital following device implantation are helpful for familiarization with the components. After the patient has returned home, a video call while the patient is doing an at-home recording can serve as a useful refresher. - Turn on the remote monitor and interrogate the device using the wand, downloading electrocorticography (ECoG) recordings that have occurred since the last interrogation to the remote monitor.
- Swipe the magnet over the INS to trigger a magnet recording.
NOTE: The magnet swipe triggers a recording with a 2:1 before:after the swipe ratio. In the case of an ECoG capture that is programmed to 240 s, this means that 160 s of data prior to the swipe and 80 s following the swipe will be stored. - Start a timer. Use the unique URL to complete a symptom survey.
- After 4 min have elapsed or the patient finishes the survey (whichever happens later), swipe the magnet over the INS again to trigger another recording.
- After at least 80 s, use the wand to interrogate the device again, transferring the data from the two magnet swipes to the remote monitor.
NOTE: Due to the limited on-board memory of the INS (up to 53 channel-minutes of data, depending on configuration13), it is desirable to immediately transfer these ECoG recordings to the remote monitor so that they are not overwritten by subsequent recordings. - Complete steps 3.1-3.6 at least twice daily.
- At least once daily, connect the remote monitor to the internet via ethernet and select Transfer Data and Synchronize on the remote monitor to send data to the cloud.
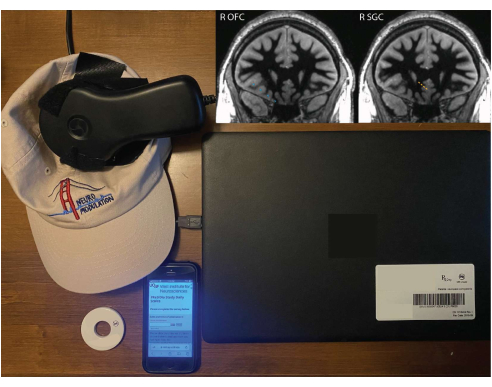
Figure 1: Patient equipment for at-home recordings. A remote monitor, wand tethered to a hat, magnet, and smartphone with REDCap survey. Inlaid images show right OFC (blue) and right SGC (orange) electrode implant locations superimposed on a white-matter nulled 1 mm isotropic T1 sequence from the preoperative magnetic resonance imaging (MRI). The depicted coronal slice is in the plane of the deepest contact, so the other contacts may not be centered on this exact slice (due to the fact that the electrode trajectory is not in the coronal plane). Please click here to view a larger version of this figure.
4. Determining a personalized biomarker
- Create a dataframe of self-reported symptom survey responses (e.g. JOVE.PR03_Symptoms.pkl).
- Calculate spectral power profiles for each channel of ECoG recording by convolving ECoG activity with a family of Morlet wavelets (40 kernels, 12 cycles, log-spaced between 1-120 Hz), creating a new dataframe (e.g., JOVE.PR03_NeuralPower.pkl).
- Associate ECoG recordings with symptom reports that occurred within a time window spanning 5 min before to 5 min after initiation of the symptom report, using the trial_id field in the NeuralPower dataframe.
- Identify symptom states (Figure 2)
- Using Python 3.10, install requirements listed in the requirements.txt file (Supplementary Folder 1) to a new environment. This can be done using pip install -r requirements.txt.
- Open JOVE-Symptom_State_and_Biomarker_Analysis.ipynb (Supplementary Folder 1) using the Jupyter Notebook.
- Verify the kernel is set to the environment into which requirements.txt were installed and run JOVE-Symptom_State_and_Biomarker_Analysis.ipynb.
NOTE: JOVE-Symptom_State_and_Biomarker_Analysis.ipynb calculates the statistical similarity between scores on patient surveys using the cosine similarity metric, which ranges from 0 (no similarity) to 1 (identical) and constructs a symptom state graph by aggregating the similarity values across all possible pairs of at-home patient symptom reports. Spectral power features for each symptom state are calculated by aggregating spectral power profiles associated with each inferred symptom state; this procedure yields a distribution of spectral power profiles linked to each symptom state (Figure 3).
- In scenarios where more than two symptom states are identified, the distributions of spectral power corresponding to the most severe symptom state and the least severe symptom state are statistically compared.
- Use a cluster-based permutation t-test to identify spectral frequencies in which the spectral power of the severe symptom state is significantly greater than the spectral power of the least severe symptom state. The range of contiguous spectral frequencies that differentiate the symptom states is considered as a single candidate biomarker.
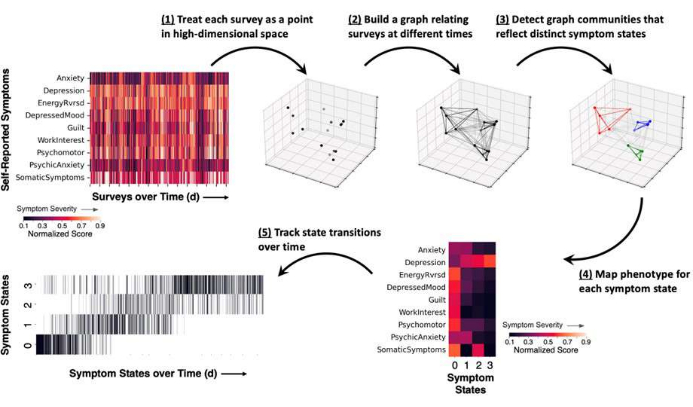
Figure 2: Schematic of the methodological approach for measuring symptom states, showing results from a representative example. Patient self-reported surveys are obtained and itemized symptom scores are normalized to a range between 0 and 1 (darker colors reflect lower symptom severity and brighter colors reflect higher symptom severity). (1) Each completed survey represents a snapshot in time of the patient's symptoms and is represented as a point (black) in high-dimensional space. (2) Time points are linked together in a symptom survey graph, which relates the cosine similarity between survey reports (lines between points). (3) Graph community detection assigns each time point to a community or symptom state (colored points and lines) based on the pattern of graph connections. (4) Symptom severity scores are averaged according to state assignment and provide a general symptom phenotype for each state. (5) The occurrence of each state may be tracked over time as a raster plot (vertical lines reflect a symptom report assigned to one state). Please click here to view a larger version of this figure.
5. Programming device detector settings
- Using the programmer, log on to the PDMS and select the correct patient, Programming, and Change Detection.
- Based on the selected personalized biomarker, select the correct channel for detecting activity.
- When creating a Pattern for the first time, first select an ECoG by clicking on one from those shown below, a channel chosen from that ECoG, and a time period highlighted.
- When creating a pattern from a preexisting one, change the channel by clicking on Pattern and selecting the Change Channel button.
- For Detect, select Rhythmic Activity, which connotes a bandpass detector.
- Click More Controls and Adv Settings. Select the desired minimum frequency and maximum frequency. Ensure the settings are as follows: Bandpass: On; Line Length: Off; Area: Off; Inversion Logic: Not Inverted.
- Program the Min Amplitude and Minimum Duration for the detector. Start with a minimum amplitude of 0.8% and a minimum duration of 0.64 s (equivalent to a Bandpass Threshold of five and Detect Analysis Window Size of 1280 ms).
NOTE: Min Amplitude is a percentage of the total signal amplitude that the detected activity must exceed. Only amplitudes above this threshold can be used for detection. Minimum Duration is how long the high amplitude signal must be sustained. This is tracked as a count of fixed-duration time bins. Specifically, x of y 128 ms time bins must meet these criteria, where x is Bandpass Threshold and y is [Detect Analysis Window Size/128], as displayed in Technical Parameters. An episode (i.e. triggering of the detector) must be exited before a subsequent trigger can occur. Some settings may be too sensitive and result in staying in one episode indefinitely, thereby preventing subsequent triggers. - Once all the detector settings are selected, click Done to close all the programming windows.
NOTE: Multiple detectors can be programmed; detection can be triggered based on AND/OR logic between these detectors. Start with one detector to understand the behavior before introducing a second detector. - With the wand placed over the patient's INS, select Review & Program and click through the confirmation messages to initialize this detector setting.
6. Titrating device detector settings
- After the device detector has been programmed and initialized on the device, conduct test recordings to assess if the sensitivity of the detector should be adjusted to achieve the desired triggering frequency. This can be achieved using Live ECoGs or the interrogation report. Adjustments to the sensitivity of the detector are determined empirically based on patient symptom reports, side effects, and outcomes. These adjustments may be needed throughout the course of treatment. Per day, 30 min of stimulation can be used as a conservative starting point to assess the clinical efficacy while also preserving battery life.
- Live ECoGs
- With the wand placed over the patient's INS, select Live ECOGs on the programmer.
- During the live recording, count the number of detections that occur for the duration of the recording. This provides an indication of how often the detector will trigger.
NOTE: Some detectors may be state-dependent, particularly detectors set to low frequencies (e.g., more active during periods of sleep or drowsiness). Thus, live recordings have limitations for estimating how often a detector may trigger. Live recordings may also suffer from electromagnetic interference (e.g., line noise) or poor wand positioning.
- Interrogation report
- Minutes to hours after setting the detector, place the wand over the patient's INS to interrogate the device.
- In the PDMS, navigate to Activity, select Event List, and click on Initial Interrogation from the time of the recording. The table at the bottom provides a list, with times, of all the detection events. This document can be exported in pdf format and parsed for quantification.
- Based on the number of detections per unit time recorded versus the desired density of stimulation, adjust the detector duration and amplitude parameters if needed. Be sure to click on Review & Program after each set of changes to initialize these on the patient's INS.
7. Programming device stimulation settings
- Using the programmer, log on to the PDMS and select the correct patient, Programming, and Change Stimulation.
- Select the desired lead contacts or Can (INS) to be anode(s) and cathode(s). Select the desired stimulation current, pulse width, duration, and frequency.
NOTE: Up to five stimulation therapies can be programmed; each therapy comprises two bursts of stimulation, which can be programmed to be the same or different. A given detector trigger can lead to a variable number of stimulation therapies, depending upon the sustained duration of the episode. Only program Therapy 1, with both bursts having the same parameters, which will lead to a consistent duration of stimulation being delivered each time the detector is triggered. In this configuration, the total duration of stimulation delivered when the detector is triggered will be the sum of Stim 1 Burst 1 and Stim 1 Burst 2. If multiple therapies are programmed and delivered, a maximum of five therapies can be delivered during a given episode. The episode must be exited and a new episode triggered in order for subsequent stimulation to be delivered. - If desired to limit the total amount of stimulation delivered per day, select a value for Therapy Limit per Day and a Therapy Limit Reset Time.
NOTE: The Therapy Limit Reset Time timezone is set to the patient's primary center. If patients have relocated, this may be different than the patient's home time zone. - Specifically, if no overnight stimulation is desired, set the Therapy Limit per Day and Therapy Limit Reset Time such that the detector achieves the therapy limit between the reset time and the patient's bedtime.
- With the wand placed over the patient's INS, select Review & Program and click through the confirmation messages to initialize the stimulation settings.
Results
Data collected and presented here are from a single patient with four-channel leads implanted in the right orbitofrontal cortex (OFC) and the right subgenual cingulate (SGC) (Figure 1). A lead with 10 mm center-to-center pitch was used for the OFC in order to target both the medial and lateral aspects, while a lead with 3.5 mm pitch was used for the SGC in order to have more spatially concentrated coverage. Four bipolar recording channels were programmed using adjacent contacts: OFC1-OFC2, O...
Discussion
Deep brain stimulation has become an established therapy for Parkinson's disease, essential tremor, dystonia, and epilepsy, and is actively being investigated in numerous other neuropsychiatric conditions26,27,28,29. The vast majority of DBS is delivered in open-loop mode, in which stimulation is delivered continuously. For symptoms which are paroxysmal in nature, continuous stimulation may...
Disclosures
ADK consults for Eisai, Evecxia Therapeutics, Ferring Pharmaceuticals, Galderma, Harmony Biosciences, Idorsia, Jazz Pharmaceuticals, Janssen Pharmaceuticals, Merck, Neurocrine Biosciences, Pernix Pharma, Sage Therapeutics, Takeda Pharmaceutical Company, Big Health, Millennium Pharmaceuticals, Otsuka Pharmaceutical, and Neurawell Therapeutics. ADK acknowledges support from Janssen Pharmaceuticals, Jazz Pharmaceuticals, Axsome Therapeutics (no. AXS-05-301), and Reveal Biosensors. KWS serves on the advisory board of Nesos. UCSF and EFC have patents related to brain stimulation for the treatment of neuropsychiatric disorders. The other authors declare no competing interests.
Acknowledgements
This work was supported by the Ray and Dagmar Dolby Family Fund through the Department of Psychiatry at UCSF (KKS, ANK, NS, JF, VRR, KWS, EFC, ADK), by a National Institutes of Health award no. K23NS110962 (KWS), NARSAD Young Investigator grant from the Brain & Behavior Research Foundation (KWS), and 1907 Trailblazer Award (KWS).
Materials
| Name | Company | Catalog Number | Comments |
| Depth Lead | Neuropace | DL-330-3.5 | 30 cm length, 3.5 mm contact spacing |
| Depth Lead | Neuropace | DL-330-10 | 30 cm length, 10 mm contact spacing |
| Depth Lead | Neuropace | DL-344-3.5 | 44 cm length, 3.5 mm contact spacing |
| Depth Lead | Neuropace | DL-344-10 | 44 cm length, 10 mm contact spacing |
| Hat with velcro | Self-assembled | NA | Optional |
| Jupyter Notebook | Project Jupyter | NA | |
| Magnet | Neuropace | M-01 | |
| Programmer | Neuropace | PGM-300 | Clinician tablet |
| Python 3.10 | Python | NA | |
| Remote Monitor | Neuropace | 5000 | Patient laptop |
| Responsive Neurostimulation System (RNS) | Neuropace | RNS-320 | |
| Wand | Neuropace | W-02 |
References
- Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 72 (6), 603-611 (2015).
- Goldstein-Piekarski, A. N., et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biological Psychiatry. 91 (6), 561-571 (2022).
- Williams, L. M. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. The Lancet Psychiatry. 3 (5), 472-480 (2016).
- Ionescu, D. F., Rosenbaum, J. F., Alpert, J. E. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues in Clinical Neuroscience. 17 (2), 111-126 (2015).
- Mayberg, H. S., et al. Deep brain stimulation for treatment-resistant depression. Neuron. 45 (5), 651-660 (2005).
- Kennedy, S. H., et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. The American Journal of Psychiatry. 168 (5), 502-510 (2011).
- Holtzheimer, P. E., et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. The Lancet Psychiatry. 4 (11), 839-849 (2017).
- Dougherty, D. D., et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biological Psychiatry. 78 (4), 240-248 (2015).
- Morishita, T., Fayad, S. M., Higuchi, M., Nestor, K. A., Foote, K. D. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 11 (3), 475-484 (2014).
- Lo, M. -. C., Widge, A. S. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. International Review of Psychiatry. 29 (2), 191-204 (2017).
- Hoang, K. B., Cassar, I. R., Grill, W. M., Turner, D. A. Biomarkers and stimulation algorithms for adaptive brain stimulation. Frontiers in Neuroscience. 11, 564 (2017).
- Little, S., et al. Adaptive deep brain stimulation in advanced Parkinson disease. Annals of Neurology. 74 (3), 449-457 (2013).
- Jarosiewicz, B., Morrell, M. The RNS system: brain-responsive neurostimulation for the treatment of epilepsy. Expert Review of Medical Devices. 18 (2), 129-138 (2021).
- Scangos, K. W., et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nature Medicine. 27 (10), 1696-1700 (2021).
- Cagle, J. N., et al. Embedded human closed-loop deep brain stimulation for Tourette syndrome: a nonrandomized controlled trial. JAMA Neurology. 79 (10), 1064-1068 (2022).
- He, S., et al. Closed-loop deep brain stimulation for essential tremor based on thalamic local field potentials. Movement Disorders. 36 (4), 863-873 (2021).
- Drysdale, A. T., et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine. 23 (1), 28-38 (2017).
- Siddiqi, S. H., et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nature Human Behaviour. 5 (12), 1707-1716 (2021).
- Ahearn, E. P. The use of visual analog scales in mood disorders: A critical review. Journal of Psychiatric Research. 31 (5), 569-579 (1997).
- Bech, P., et al. The Hamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatrica Scandinavica. 63 (3), 290-299 (1981).
- Khambhati, A. N., Sizemore, A. E., Betzel, R. F., Bassett, D. S. Modeling and interpreting mesoscale network dynamics. NeuroImage. 180, 337-349 (2018).
- Zamani Esfahlani, F., Bertolero, M. A., Bassett, D. S., Betzel, R. F. Space-independent community and hub structure of functional brain networks. NeuroImage. 211, 116612 (2020).
- Kleen, J. K., Rao, V. R. Managing neurostimulation for epilepsy. Deep Brain Stimulation Management. , 177-197 (2022).
- Krucoff, M. O., Wozny, T. A., Lee, A. T., Rao, V. R., Chang, E. F. Operative technique and lessons learned from surgical implantation of the NeuroPace Responsive Neurostimulation® system in 57 consecutive patients. Operative Neurosurgery. 20 (2), E98-E109 (2021).
- Harris, P. A., et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 95, 103208 (2019).
- Krauss, J. K., et al. Technology of deep brain stimulation: current status and future directions. Nature Reviews Neurology. 17 (2), 75-87 (2021).
- Dougherty, D. D. Deep brain stimulation: clinical applications. The Psychiatric Clinics of North America. 41 (3), 385-394 (2018).
- Drobisz, D., Damborská, A. Deep brain stimulation targets for treating depression. Behavioural Brain Research. 359, 266-273 (2019).
- Lee, D. J., Lozano, C. S., Dallapiazza, R. F., Lozano, A. M. Current and future directions of deep brain stimulation for neurological and psychiatric disorders. Journal of Neurosurgery. 131 (2), 333-342 (2019).
- Sun, F. T., Morrell, M. J. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 11 (3), 553-563 (2014).
- Malone, D. A., et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biological Psychiatry. 65 (4), 267-275 (2009).
- Zuo, X. -. N., Xu, T., Milham, M. P. Harnessing reliability for neuroscience research. Nature Human Behaviour. 3 (8), 768-771 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved