A subscription to JoVE is required to view this content. Sign in or start your free trial.
Viability Assay of Trichoderma stromaticum Conidia Inside Human Peripheral Blood Mononuclear-Derived Macrophages
* These authors contributed equally
In This Article
Summary
The technique involving the phagocytosis of fungal conidia by macrophages is widely used for studies evaluating the modulation of the immune responses against fungi. The purpose of this manuscript is to present a method for evaluating the phagocytosis and clearance abilities of human peripheral blood mononuclear-derived macrophages stimulated with Trichoderma stromaticum conidia.
Abstract
Macrophages represent a crucial line of defense and are responsible for preventing the growth and colonization of pathogens in different tissues. Conidial phagocytosis is a key process that allows for the investigation of the cytoplasmic and molecular events involved in macrophage-pathogen interactions, as well as for the determination of the time of death of internalized conidia. The technique involving the phagocytosis of fungal conidia by macrophages is widely used for studies evaluating the modulation of the immune responses against fungi. The evasion of phagocytosis and escape of phagosomes are mechanisms of fungal virulence. Here, we report the methods that can be used for the analysis of the phagocytosis, clearance, and viability of T. stromaticum conidia, a fungus which is used as a biocontrol and biofertilizer agent and is capable of inducing human infections. The protocol consists of 1) Trichoderma culture, 2) washing to obtain conidia, 3) the isolation of peripheral blood mononuclear cells (PBMCs) using the polysucrose solution method and the differentiation of the PBMCs into macrophages, 4) an in vitro phagocytosis method using round glass coverslips and coloration, and 5) a clearance assay to assess the conidia viability after conidia phagocytosis. In summary, these techniques can be used to measure the fungal clearance efficiency of macrophages.
Introduction
The Trichoderma genus (Order: Hypocreales, Family: Hypocreaceae) is composed of ubiquitous, saprophytic fungi that are parasites of other fungal species and are capable of producing a range of commercially useful enzymes1. These fungal species are used for the production of heterologous proteins2, the production of cellulose3, ethanol, beer, wine, and paper4, in the textile industry5, food industry6, and in agriculture as biological control agents7,8. In addition to the industrial interest in these fungal species, the increasing number of infections in humans has given some Trichoderma species the status of opportunistic pathogens9.
Trichoderma spp. grow rapidly in culture, with initially white and cottony colonies that turn greenish yellow to dark green10. They are adapted to live in a wide range of pH and temperature conditions, and the opportunistic species are able to survive at physiological pH and temperatures and, thus, colonize different human tissues11,12,13. Importantly, the rise in the infection rate of Trichoderma spp. may be associated with virulence factors, and these are not well studied. In addition, studies focusing on understanding the immune response against opportunistic Trichoderma species are still rare.
During an infection, along with neutrophils, macrophages represent the line of defense responsible for phagocytosis, and, thus, prevent the growth and colonization of pathogens in different tissues. Using pattern recognition receptors, such as Toll-like receptors and C-type lectin receptors, macrophages phagocytose fungi and process them into phagolysosomes, thus promoting a respiratory burst, the release of pro-inflammatory cytokines, and the destruction of the phagocytosed microorganisms14. The mechanism of phagocytosis, however, can be affected and evaded by different microbial strategies, such as the size and shape of the fungal cells; the presence of capsules that hinder phagocytosis; decreasing the number of phagocytosis-inducing receptors; the remodeling of the structure of actin fibers in the cytoplasm; hindering the formation of pseudopodia; and phagosome or phagolysosome escape after the phagocytosis process14.
Many pathogens, including Cryptococcus neoformans, use macrophages as a niche to survive in the host, disseminate, and induce infection15. The phagocytosis and clearance assay is used to evaluate the immune response against pathogens and to identify the microbial strategies employed to evade the innate immune system15,16,17. This type of technique can also be used to examine the differential kinetics of phagocytosis, delayed phagosome acidification, and oxidative burst that result in reduced fungal killing18.
Different methods can be used to evaluate phagocytosis, fungal survival, and the evasion of the phagosome maturation process. These include fluorescence microscopy, which is used to observe phagocytosis, the cellular location, and the molecules produced during phagocytosis19; flow cytometry, which provides quantitative data on phagocytosis and is used to evaluate the different markers involved in the process20,21; intravital microscopy, which is used to assess microbial capture and phagosome maturation22; antibody-mediated phagocytosis, which is used to assess the specificity of the phagocytosis process for a pathogen23; and others24,25,26,27.
The protocol presented here employs a common, low-cost, and direct method using an optical microscope and plate growth assay to assess the phagocytosis and killing of fungal conidia. This protocol will provide the readers with step-by-step instructions for performing the phagocytosis and clearance assay using human peripheral blood mononuclear-derived macrophages exposed to T. stromaticum. PBMCs were used because Trichoderma conidia are applied as a biocontrol against phytopathogens and a biofertilizer for plant crops worldwide and have caused several human infections, called Trichodermosis. Besides that, there are only two previous works focusing on the interaction between Trichoderma conidia and the human immune system, in which we examined neutrophils28 and autophagy in macrophages29. This article shows first how the phagocytosis of the conidia of T. stromaticum by PBMC-derived macrophages can be studied, and then how the viability of the engulfed conidia can be assessed using simple microscopy-based techniques. This protocol may further facilitate investigations on macrophage-associated immune response or immune system modulation-related mechanisms.
Protocol
Ethical considerations and human subjects
All experiments with humans described in this study were conducted according to the Declaration of Helsinki and Brazilian Federal laws and approved by the State University of Santa Cruz's Ethics Committee (project identification code: 550.382/ 2014).
Human peripheral blood was collected from healthy volunteers from Ilhéus city, Bahia, Brazil, not exposed to occupational activities related to the studied fungus. Individuals with reported health medical conditions or using medication were excluded. All subjects voluntarily agreed to participate and signed an informed consent form before their inclusion in this study.
1. Preparation of the reagents and solutions
NOTE: Prepare the following reagents and solutions before proceeding. See the Table of Materials (TOM) for the reagent and material supplier information.
- Prepare a balanced sterile phosphate-buffered saline solution 1x (PBS).
NOTE: In this protocol, endotoxin-free PBS was not used. - Prepare potato dextrose agar (PDA) medium, and pour 20 mL into each Petri dish for the T. stromaticum culture. Prepare six plates with PDA for each donor, and use one plate for each condition: 3 h, 24 h, 48 h, 72 h, 96 h, and 120 h. Additionally, use three plates for each control: three for the Trichoderma control and three for the R10 medium control. Use Petri dishes of 60 mm x 15 mm or 100 mm x 15 mm for optimal conidia recovery.
- Sterilize 100% glycerol.
- Prepare an R10 medium for the cell culture: RPMI medium supplemented with 10% fetal bovine serum and 1% antibiotic (penicillin/streptomycin).
- Sterilize distilled water for the cell lysis.
- Prepare water at pH 7.0 for the coloration process.
NOTE: If necessary, adjust the pH using a pH meter and solutions of 0.1 M/L NaOH and 0.1 M/L HCl. - Sterilize round glass coverslip (13 mm) and tweezers.
2. Trichoderma stromaticum culture and processing
- Preparation of a mother stock (M1) of T. stromaticum:
NOTE: A mother stock (M1) is required to ensure that all the experiments are done from the culture of the same fresh generation (F1).- Grow a T. stromaticum inoculum in Petri dishes with PDA at 28 °C in the dark for 7-10 days until the conidia are observed and the culture turns green.
- Wash the culture of T. stromaticum. Add 3-5 mL of PBS to the culture surface using a pipette. Collect the PBS from the plate, and again dispense it over the culture to gradually remove the conidia using the pipette. Repeat this process as much as necessary until the suspension turns dark green.
NOTE: Alternatively, gentle washing of the culture by adding 3-5 mL of PBS to the plate followed by performing a circular movement is suggested until the suspension turns green to recover the conidia. With repeated pipetting, more conidia can be collected. - Transfer the recovered suspension from the plate into a 15 mL sterile tube with a pipette. Repeat steps 2.1.2-2.1.3 as many times as necessary to obtain more conidia.
- Centrifuge the suspension at 1,160 x g for 5 min at 12 °C, and resuspend the pellet in 5 mL of PBS. Repeat this step (2.1.4) three times to obtain a hyphae-free suspension.
NOTE: To resuspend the pellet properly, after centrifugation, brief vortexing is recommended. - Resuspend the final pellet in 2-3 mL of PBS, and count the conidia in a Neubauer chamber using a dilution of 1:100 or 1:1,000.
NOTE: In optimal conditions, a final concentration of 2 x 108 conidia/mL can be recovered from the culture. Assessing the conidia viability using the trypan blue exclusion method is optional. - Aliquot the suspension into 1.5 mL sterile tubes by adding 0.5 mL of the conidia suspension (from step 2.1.5) to each tube. Then, add 0.5 mL of sterile 100% glycerol to obtain M1 stocks. Vortex briefly, identify the tubes, and store at −20 °C or −80 °C.
- Culture of T. stromaticum and obtaining conidia for the experiment
- Plate 10 µL of the M1 suspension (prepared in step 2.1) in PDA at 28 °C in the dark for 7-10 days until the conidia are observed and the culture turns green to obtain the fresh F1 generation.
NOTE: To ensure a fresh T. stromaticum culture for the experiment, assess the time required for fungus growth and the time required for macrophage differentiation. - Collect the conidia, repeating steps 2.1.2-2.1.4.
- Resuspend the final pellet in 2-3 mL of PBS. Assess the conidia viability using the trypan blue exclusion method, or count the conidia as mentioned in step 2.1.5.
- Plate 10 µL of the M1 suspension (prepared in step 2.1) in PDA at 28 °C in the dark for 7-10 days until the conidia are observed and the culture turns green to obtain the fresh F1 generation.
3. Peripheral blood mononuclear cell (PBMC) isolation
NOTE: The PBMCs are obtained by the density barrier method using a polysucrose solution of density 1.077 g/mL30.
- Aliquot 15 mL of the polysucrose solution to one 50 mL tube for every donor sample that will be collected.
- Collect 20 mL of blood from each donor using three to four EDTA tubes. Recruit a minimum of four donors per experiment. Do not pool the blood of the donors; instead, use each sample separately as a biological replicate. For each donor, transfer the blood to one 50 mL tube, and add PBS to obtain a final volume of 35 mL.
- Transfer the blood suspension (step 3.2) slowly along the wall of the tube containing the polysucrose solution (step 3.1) to form a biphasic suspension.
NOTE: To prevent the mixing of the blood with the polysucrose solution, add the blood slowly. The use of a minimum of 7 mL of blood from each donor for the PBMC isolation is recommended. - Immediately centrifuge the tube containing the biphasic suspension at 1,600 x g for 30 min at 24 °C. Program the centrifugation of the PBMCs: use acceleration 1 and deceleration 0 to avoid an abrupt stop of the centrifugation.
- Observe the formation of a white ring containing the PBMCs after centrifugation. The red blood cells and granulocytes reside in the lower layer, the next layer contains the polysucrose solution, and the PBMCs reside in the white ring between the polysucrose solution and the plasma (upper layer). Collect the white ring by aspirating gently using a pipette or a sterile Pasteur pipette so as not to catch red blood cells, plasma, or polysucrose solution, and transfer to one 15 mL tube.
- Add PBS to the 15 mL tube containing the PBMCs to obtain a final volume of 10 mL. Homogenize the suspension, and centrifuge the tube at 1,600 x g for 30 min at 24 °C.
- Wash the cells three times at 1,600 x g for 5 min at 24 °C with 10 mL of PBS. Then, resuspend the final pellet in 2 mL of R10 medium.
- Assess the cell viability using the trypan blue exclusion method, and count the cells in a Neubauer chamber.
4. Phagocytosis kinetics
NOTE: The human peripheral blood mononuclear-derived macrophages are treated with T. stromaticum conidia at a multiplicity of infection (MOI) of 1:10 or only R10 medium as a control. The multiplicity of infection (MOI) represents the ratio of the infectious agents added to the infection targets and is presented as absolute numbers: MOI = agents:targets31. Here, we use 1 T. stromaticum conidia (agent) for 10 macrophages (targets). Then, the cells are incubated at 37 °C and 5% CO2 for different periods of time (3 h, 24 h, 48 h, 72 h, 96 h, and 120 h). Next, a round glass coverslip is used to visualize the adherent macrophages involved in the phagocytosis process under a microscope. An experimental design figure is provided (Figure 1).
NOTE: The use of 24-well plates is suggested.
- Treatment of human-derived macrophages with T. stromaticum conidia
- Add a sterile round glass coverslip (13 mm) to each well using sterile tweezers.
- Adjust the volume of the cell suspension obtained in steps 3.7-3.8 to plate 8 x 105 cells per well to a final volume of 1 mL with R10 medium. Use an inverted microscope to assess the cell morphology.
NOTE: The cells must be suspended in the medium and have a rounded morphology. - Incubate the cells at 37 °C under a 5% CO2 atmosphere for 7 days, and replace the medium with fresh R10 medium every 2 days for the differentiation of the monocytes into macrophages32. Observe the cell morphology using an inverted microscope. The macrophages will present spindled morphology, with flatted and extended shapes; an increased cytoplasmatic ratio and the presence of pseudopodia can also be observed. Look for adherent mononuclear-derived macrophages.
NOTE: A very small proportion of undifferentiated monocytes may remain suspended in the medium. - Remove the medium of each well using a pipette, and wash the cells with 1 mL of PBS to remove debris and non-adherent cells.
- Use the suspension prepared in step 2.2 to prepare a new suspension of T. stromaticum conidia at a final concentration of 8 x 104 conidia/mL in R10 medium.
- Add 1 mL of the conidia suspension prepared in step 4.1.5 to each treatment well (3 h, 24 h, 48 h, 72 h, 96 h, and 120 h) to obtain a MOI of 1:10. To the controls, add only 1 mL of R10 medium to the well.
- Challenge the macrophages for 3 h with T. stromaticum conidia at 37 °C under a 5% CO2 atmosphere. After 3 h, remove the medium, and wash the cells to remove the non-phagocytosed conidia.
- Add 1 mL of R10 medium to each well, and incubate the plate for different times (3 h, 24 h, 48 h, 72 h, 96 h, and 120 h) at 37 °C under a 5% CO2 atmosphere.
- Coloration and analysis
- Remove the medium from each well after the corresponding incubation (step 4.1.8) is complete, and add 1 mL of PBS to that well.
- Remove the round glass coverslips from the wells using sterile tweezers and a needle for coloring with a staining kit.
NOTE: Alternatively, staining can be done using May Grünwald-Giemsa stain33. - To each of the round glass coverslips, add stain fixator (5 s), Dye 1 (5 s), Dye 2 (2 s), and buffered water pH 7.0 (5 s), which are the components of the staining kit. Wait until they are dry, and fix the coverslips on a glass slide using mounting agent.
- Quantify the result: Count a total of 100 macrophages (Mø) for each time condition. Using an optical microscope, count the number of macrophages with phagocytosed conidia using a 100x objective and immersion oil (equation [1]).
 (1)
(1) - Obtain the number of conidia phagocytosed per macrophage: Correlate the conidia present in 100 macrophages divided by the number of macrophages with at least one phagocytosed conidium (equation [2])34.
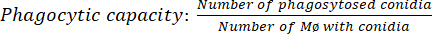 (2)
(2)
NOTE: To evaluate and count the conidia, the ImageJ software can be used35. - Photograph the phagocytosis from each sample and time condition using 40x and 100x objectives to present the results.
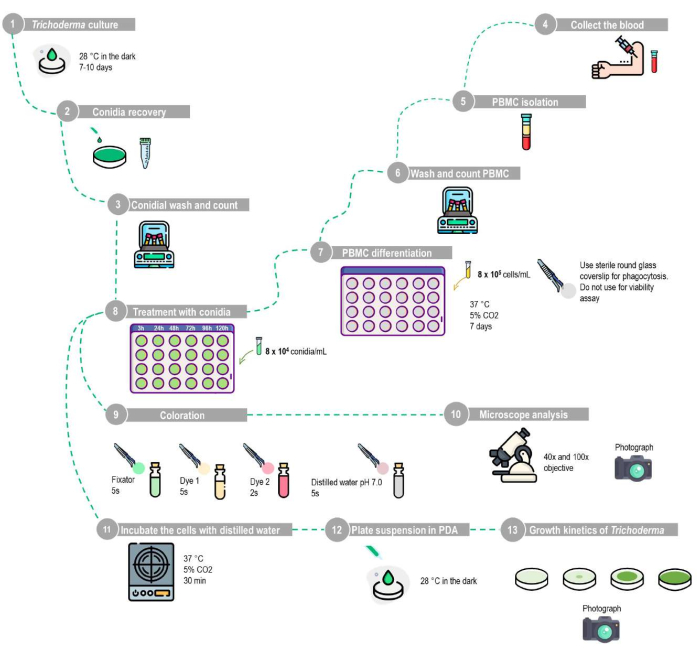
Figure 1: Schematic representation of the phagocytosis and conidial viability assay using human peripheral blood mononuclear-derived macrophages. (1-10) Phagocytosis assay: The T. stromaticum must be grown in PDA, and the conidia are recovered by washing the plate with PBS. The PBMCs should be isolated by the density barrier method using the described protocol, cultured in 24-well plates with sterile round glass coverslips for 7 days for differentiation into macrophages, and then treated with an MOI of 1:10 with different time intervals. The round glass coverslips are removed and stained with the staining kit, and the results are analyzed using light microscopy. (1-8,11-13) Conidia viability assay: After isolation, the PBMCs are cultured in 24-well plates without round glass coverslips for 7 days for differentiation into macrophages and then treated with an MOI of 1:10 for different time intervals. The cells must be lysed by incubation with distilled water; the suspension is then centrifuged, and then the resuspended pellet is plated in PDA to analyze the growth kinetics of Trichoderma. This figure was designed using a database of images. Please click here to view a larger version of this figure.
5. Conidial viability assay after phagocytosis
NOTE: An experimental design figure is provided (Figure 1).
NOTE: Use 24-well plates.
- Challenge the human-derived macrophages with T. stromaticum conidia.
- Repeat steps 4.1.2-4.1.8 to differentiate the monocytes, and treat the macrophages with T. stromaticum conidia.
NOTE: Do not use round glass coverslips in the wells. Use the same time intervals used for assaying the phagocytosis kinetics so that the phagocytosis can be correlated with the conidial viability. - Evaluate the impact of the R10 medium on the T. stromaticum growth by using wells with the suspension prepared in step 4.1.5 (R10 medium + conidia) as a control.
- Repeat steps 4.1.2-4.1.8 to differentiate the monocytes, and treat the macrophages with T. stromaticum conidia.
- Conidial viability assay
- Remove the medium after each incubation period (3 h, 24 h, 48 h, 72 h, 96 h, and 120 h), and wash the cells twice with PBS to remove the conidia that have not been phagocytosed.
NOTE: The control containing R10 medium + conidia should not be washed. - Add 0.5 mL of sterile distilled water to each well, and incubate the plate at 37 °C and 5% CO2 for 30 min. After this time, observe the cell morphology under an inverted microscope to ensure the cells have been lysed.
- Collect the suspension, and transfer it to 1.5 mL tubes. Centrifuge the suspension using a mini centrifuge at 6,000 x g for 5 min at room temperature.
- Discard the supernatant carefully, resuspend the pellet in 10 µL of sterile distilled water, and inoculate 10 µL of the suspension into the PDA-containing plate at 28 °C in the dark.
- Prepare a positive control for T. stromaticum growth: Use the suspension of T. stromaticum conidia (step 2.2) to prepare a new suspension with a final concentration of 8 x 106 conidia/mL. Plate 10 µL of this suspension in PDA at 28 °C in the dark.
NOTE: 10 µL of this suspension will contain the same number of conidia (8 x 104) that was used to infect the blood mononuclear-derived macrophages. The difference between this positive control and the one mentioned in step 5.1.2 is that the control mentioned above in step 5.1.2 is used to evaluate the possible impact of the R10 medium on Trichoderma growth during the time of phagocytosis. This positive control in step 5.2.5 using the suspension of T. stromaticum conidia collected in PBS represents a control for Trichoderma growth without the influence of R10 medium to reflect how the fungus grows naturally. Using this control, it is possible to evaluate if the R10 medium influences the Trichoderma growth. - Observe the growth kinetics of T. stromaticum in PDA every day, and photograph the culture.
- Analysis: Two points can be used to evaluate the results: 1) the time (in days) required for the culture to occupy the entire culture plate, and 2) the time (in days) until the appearance of the characteristic green pigmentation of the T. stromaticum conidia in culture.
- Remove the medium after each incubation period (3 h, 24 h, 48 h, 72 h, 96 h, and 120 h), and wash the cells twice with PBS to remove the conidia that have not been phagocytosed.
6. Statistical analysis
- Use a Kruskal-Wallis test to determine statistically significant differences between the 8 groups/treatments. Consider p < 0.05 as statistically significant. All the data are presented as means ± standard deviation (SD). In the figures, # indicates statistical significance at p < 0.05.
Results
The technique involving the phagocytosis of fungal conidia by macrophages is widely used for studies evaluating the modulation of the immune responses against fungi. We used the phagocytosis of T. stromaticum conidia to assess the viability of the conidia after phagocytosis, since the evasion of phagocytosis and the escape of phagosomes are mechanisms of fungal virulence. Researchers should perform these techniques as one of the first assays when investigating a species of clinical interest.
Discussion
For several fungal pathogens including Aspergillus fumigatus, Cryptococcus, Candida albicans, and others, conidial or yeast phagocytosis is a key process that allows for the investigation of the cytoplasmic and molecular events in macrophage-pathogen interactions, as well as for the determination of the time of death of the internalized conidia14,39,40. Phagocytosis is the key process in the T...
Disclosures
All the authors declare no conflicts of interest.
Acknowledgements
This work was supported by the following Brazilian financing institutions: Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) with grants RED0011/2012 and RED008/2014. U.R.S., J.O.C., and M.E.S.M. acknowledge the scholarship granted by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and FAPESB, respectively.
Materials
| Name | Company | Catalog Number | Comments |
| 15 mL centrifuge tubes | Corning | CLS431470 | 15 mL centrifuge tubes, polypropylene, conical bottom with lid, individually sterile |
| 24-Well Flat Bottom Cell Culture Plate | Kasvi | K12-024 | Made of polystyrene with alphanumeric identification; The Cell Culture Plate is DNase, RNase and pyrogen-free and free of cytotoxic substances; Sterilized by gamma radiation; |
| Cell culture CO2 incubator | Sanyo | 303082 | A CO2 incubator serves to create and control conditions similar to a human body, thus allowing the in vitro growth and proliferation of different cell types. |
| Centrifuge Microtube (eppendorf type) 1.5 mL | Capp | 5101500 | Made from polypropylene, with a cap attached to the tube for opening and closing with just one hand. It has a polished interior against protein adhesion and for sample visibility, being free of DNase, RNase and Pyrogens |
| Circular coverslip 15 mm | Olen | K5-0015 | Circular coverslips are used for microscopy techniques in cell culture. Made of super transparent translucent glass; with thickness of 0.13 mm |
| Class II Type B2 (Total Exhaust) Biosafety Cabinets | Esco Lifesciences group | 2010274 | Airstream Class II Type B2 Biosafety Cabinets (AB2) provide product, operator and environmental protection and are suitable for work with trace amounts of toxic chemicals and agents assigned to biological safety levels I, II or III. In a Class II Type B2 cabinet, all inflow and downflow air is exhausted after HEPA/ULPA filtration to the external environment without recirculation across the work surface. |
| Dextrose Potato Agar medium | Merck | 145 | Potato Dextrose Agar is used in the cultivation and enumeration of yeasts and fungi |
| EDTA vacuum blood collection tube | FirstLab | FL5-1109L | EDTA is the recommended anticoagulant for hematology routines as it is the best anticoagulant for preserving cell morphology. |
| Entellan | Merck | 1.07961 | Fixative agent; Entellan is a waterless mounting medium for permanent mounting for microscopy. |
| Fetal Bovine Serum | Gibco | A2720801 | Fetal bovine serum (FBS) is a universal growth supplement of cell and tissue culture media. FBS is a natural cocktail of most of the factors required for cell attachment, growth, and proliferation, effective for most types of human and animal (including insect) cells. |
| Flaticon | database of images | ||
| Glycerol | Merck | 24900988 | The cryoprotectant agent glycerol is used for freezing cells and spores |
| Histopaque-1077 | polysucrose solution | ||
| Image J | Image analysis software | ||
| Microscopy slides | Precision | 7105 | Slide for Microscopy 26 x 76 mm Matte Lapped Thickness 1.0 to 1.2 mm. Made of special optical glass and packaged with silk paper divider with high quality transparency free of imperfections |
| Mini centrifuge | Prism | C1801 | The Prism Mini Centrifuge was designed to be extremely compact with an exceptionally small footprint. Includes 2 interchangeable quick-release rotors that spin up to 6000 rpm. An electronic brake provides quick deceleration and the self-opening lid allows easy access to the sample, reducing handling time. |
| Neubauer chamber | Kasvi | K5-0011 | The Neubauer Counting Chamber is used for counting cells or other suspended particles. |
| Panoptic fast | Laborclin | 620529 | Laborclin's panoptic fast c is a kit for quick staining in hematology |
| Penicillin/Streptomycin Solution - 10,000U | LGC- Biotechnology | BR3011001 | antibiotic is used in order to avoid possible contamination by manipulation external to the laminar flow. |
| Petri dish 90 x 15 mm Smooth | Cralplast | 18248 | Disposable Petri dish; Made of highly transparent polystyrene (PS); flat bottom; Smooth;Size: 90 x 15 mm. |
| Phosphate buffered saline (PBS) | thermo fisher Scientific | 10010001 | PBS is a water-based saline solution with a simple formulation. It is isotonic and non-toxic to most cells. It includes sodium chloride and phosphate buffer and is formulated to prevent osmotic shock while maintaining the water balance of living cells. |
| Pipette Pasteur 3 mL Sterile | Accumax | AP-3-B-S | STERILE ACCUMAX PASTEUR 3 ML PIPETTE with 3 mL capacity, made of transparent low-density polyethylene (LDPE) and individually sterile |
| Refrigerated Centrifuge | Thermo Scientific | TS-HM16R | The Thermo Scientific Heraeus Megafuge 16R Refrigerated Centrifuge is a refrigerated centrifuge with the user-friendly control panel makes it easy to pre-set the speed, RCF value, running time, temperature, and running profile. The Megafuge 16R can reach maximum speeds of 15,200 RPM and maximum RCF of 25,830 x g. |
| RPMI-1640 Medium | Merck | MFCD00217820 | HEPES Modification, with L-glutamine and 25 mM HEPES, without sodium bicarbonate, powder, suitable for cell culture |
| The single channel micropipettes | Eppendorf | Z683809 | Single-channel micropipettes are used to accurately transfer and measure very small amounts of liquids. |
| Tip for Micropipettor | Corning | 4894 | Capacity of 10 µL and 1,000 µL Autoclavable |
| Triocular inverted microscope | LABOMED | VU-7125500 | It allows you to observe cells inside tubes and bottles, without having to open them, thus avoiding contamination problems. |
References
- Samuels, G. J. Trichoderma: A review of biology and systematics of the genus. Mycological Research. 100 (8), 923-935 (1996).
- Nevalainen, H., Peterson, R., Gupta, V. K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R. S., Druzhinina, I., Tuohy, M. G. Chapter 7 - Heterologous expression of proteins in Trichoderma. Biotechnology and Biology of Trichoderma. , (2014).
- Do Vale, L. H. F., Filho, E. X. F., Miller, R. N. G., Ricart, C. A. O., de Sousa, M. V., Gupta, V. K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R. S., Druzhinina, I., Tuohy, M. G. Chapter 16 - Cellulase systems in Trichoderma: An overview. Biotechnology and Biology of Trichoderma. , (2014).
- Ferreira, N. L., Margeot, A., Blanquet, S., Berrin, J. G., Gupta, V. K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R. S., Druzhinina, I., Tuohy, M. G. Chapter 17 - Use of cellulases from Trichoderma reesei in the twenty-first century part I: Current industrial uses and future applications in the production of second ethanol generation. Biotechnology and Biology of Trichoderma. , (2014).
- Puranen, T., Alapuranen, M., Vehmaanperä, J., Gupta, V. K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R. S., Druzhinina, I., Tuohy, M. G. Chapter 26 - Trichoderma enzymes for textile industries. Biotechnology and Biology of Trichoderma. , (2014).
- Kunamneni, A., Plou, F. J., Alcalde, M., Ballesteros, A., Gupta, V. K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R. S., Druzhinina, I., Tuohy, M. G. Chapter 24 - Trichoderma enzymes for food industries. Biotechnology and Biology of Trichoderma. , (2014).
- Mukherjee, P. K., Horwitz, B. A., Herrera-Estrella, A., Schmoll, M., Kenerley, C. M. Trichoderma research in the genome era. Annual Review of Phytopathology. 51 (1), 105-129 (2013).
- Mukherjee, M., et al. Trichoderma-plant-pathogen interactions: Advances in genetics of biological control. Indian Journal of Microbiology. 52 (4), 522-529 (2012).
- dos Santos, U. R., dos Santos, J. L. Trichoderma after crossing kingdoms: Infections in human populations. Journal of Toxicology and Environmental Health, Part B. 26 (2), 97-126 (2023).
- Asis, A., et al. Identification patterns of Trichoderma strains using morphological characteristics, phylogenetic analyses and lignocellulolytic activities. Molecular Biology Reports. 48 (4), 3285-3301 (2021).
- Antal, Z., et al. Comparative study of potential virulence factors in human pathogenic and saprophytic Trichoderma longibrachiatum strains. Acta Microbiologica et Immunologica Hungarica. 52 (3-4), 341-350 (2005).
- Hatvani, L., Manczinger, L., Vágvölgyi, C., Kredics, L., Mukherjee, P. K., Horwitz, B. A., Singh, U. S., Mukherjee, M., Schmoll, M. Trichoderma as a human pathogen. Trichoderma: Biology and Applications. , (2013).
- Kredics, L., et al. Clinical importance of the genus Trichoderma: A review. Acta Microbiologica et Immunologica Hungarica. 50 (2-3), 105-117 (2003).
- Erwig, L. P., Gow, N. A. R. Interactions of fungal pathogens with phagocytes. Nature Reviews Microbiology. 14 (3), 163-176 (2016).
- Nicola, A. M., Casadevall, A. In vitro measurement of phagocytosis and killing of Cryptococcus neoformans by macrophages. Methods in Molecular Biology. 844, 189-197 (2012).
- Medina, E., Goldmann, O. In vivo and ex vivo protocols for measuring the killing of extracellular pathogens by macrophages. Current Protocols in Immunology. , 1-17 (2011).
- Drevets, D. A., Canono, B. P., Campbell, P. A. Measurement of bacterial ingestion and killing by macrophages. Current Protocols in Immunology. 109, 1-17 (2015).
- Gresnigt, M. S., et al. Differential kinetics of Aspergillus nidulans and Aspergillus fumigatus phagocytosis. Journal of Innate Immunity. 10 (2), 145-160 (2018).
- Steinberg, B. E., Grinstein, S. Analysis of macrophage phagocytosis: Quantitative assays of phagosome formation and maturation using high-throughput fluorescence microscopy. Methods in Molecular Biology. 531, 45-56 (2009).
- Yan, Q., Ahn, S. H., Fowler, V. G. Macrophage phagocytosis assay of Staphylococcus aureus by flow cytometry. Bio-Protocol. 5 (4), 1406 (2015).
- Marr, K. A., Koudadoust, M., Black, M. Early events in macrophage killing of Aspergillus fumigatus conidia New flow cytometric viability assay. Clinical and Diagnostic Laboratory Immunology. 8 (6), 1240-1247 (2001).
- Surewaard, B. G. J., Kubes, P. Measurement of bacterial capture and phagosome maturation of Kupffer cells by intravital microscopy. Methods. 128, 12-19 (2017).
- Siggins, M. K., et al. Differential timing of antibody-mediated phagocytosis and cell-free killing of invasive African Salmonella allows immune evasion. European Journal of Immunology. 44 (4), 1093-1098 (2014).
- Cannon, G. J., Swanson, J. A. The macrophage capacity for phagocytosis. Journal of Cell Science. 101 (4), 907-913 (1992).
- Harvath, L., Terle, D. A. Assay for phagocytosis. Methods in Molecular Biology. 115, 281-290 (1999).
- dos Santos, A. G., et al. Trichoderma asperelloides spores downregulate dectin1/2 and TLR2 receptors of mice macrophages and decrease Candida parapsilosis phagocytosis independent of the M1/M2 polarization. Frontiers in Microbiology. 8, 1681 (2017).
- Souza, J. A. M., et al. Characterization of Aspergillus fumigatus extracellular vesicles and their effects on macrophages and neutrophils functions. Frontiers in Microbiology. 10, 2008 (2019).
- Oliveira-Mendonça, L. S., et al. Inhibition of extracellular traps by spores of Trichoderma stromaticum on neutrophils obtained from human peripheral blood. Molecular Immunology. 141, 43-52 (2022).
- Oliveira-Mendonça, L. S., et al. Trichoderma stromaticum spores induce autophagy and downregulate inflammatory mediators in human peripheral blood-derived macrophages. Current Research in Microbial Sciences. 3, 100145 (2022).
- Johnston, L., Harding, S. A., La Flamme, A. C. Comparing methods for ex vivo characterization of human monocyte phenotypes and in vitro responses. Immunobiology. 220 (12), 1305-1310 (2015).
- Abedon, S. T., Bartom, E., Maloy, S., Hughes, K. Multiplicity of infection. Brenner's Encyclopedia of Genetics. Second Edition. , (2013).
- Rios, F. J., Touyz, R. M., Montezano, A. C. Isolation and differentiation of human macrophages. Methods in Molecular Biology. 1527, 311-320 (2017).
- Lombard, Y., Giaimis, J., Makaya-Kumba, M., Fonteneau, P., Poindron, P. A new method for studying the binding and ingestion of zymosan particles by macrophages. Journal of Immunological Methods. 174 (1-2), 155-165 (1994).
- Ghoneum, M., Gollapudi, S. Phagocytosis of Candida albicans by metastatic and non metastatic human breast cancer cell lines in vitro. Cancer Detection and Prevention. 28 (1), 17-26 (2004).
- Nunes, J. P. S., Dias, A. A. M. ImageJ macros for the user-friendly analysis of soft-agar and wound-healing assays. BioTechniques. 62 (4), 175-179 (2017).
- Alves-Filho, E. R., et al. The biocontrol fungus Trichoderma stromaticum downregulates respiratory burst and nitric oxide in phagocytes and IFN-gamma and IL-10. Journal of Toxicology and Environmental Health - Part A: Current Issues. 74 (14), 943-958 (2011).
- Slesiona, S., et al. Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS One. 7 (2), 31223 (2012).
- Johnston, S. A., May, R. C. Cryptococcus interactions with macrophages: Evasion and manipulation of the phagosome by a fungal pathogen. Cellular Microbiology. 15 (3), 403-411 (2013).
- Alonso, M. F., et al. The nature of the fungal cargo induces significantly different temporal programmes of macrophage phagocytosis. The Cell Surface. 8, 100082 (2022).
- Brakhage, A. A., Bruns, S., Thywissen, A., Zipfel, P. F., Behnsen, J. Interaction of phagocytes with filamentous fungi. Current Opinion in Microbiology. 13 (4), 409-415 (2010).
- Dos Santos, U. R., et al. Exposition to biological control agent Trichoderma stromaticum increases the development of cancer in mice injected with murine melanoma. Frontiers in Cellular and Infection Microbiology. 10, 252 (2020).
- Wang, G., et al. Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation. Carbohydrate Polymers. 149, 112-120 (2016).
- Xu, Y., et al. Exopolysaccharide from Trichoderma pseudokoningii promotes maturation of murine dendritic cells. International Journal of Biological Macromolecules. 92, 1155-1161 (2016).
- Schmoll, M., Esquivel-Naranjo, E. U., Herrera-Estrella, A. Trichoderma in the light of day - Physiology and development. Fungal Genetics and Biology. 47 (11), 909-916 (2010).
- Zhang, G., Li, D. Trichoderma longibrachiatum-associated skin inflammation and atypical hyperplasia in mouse. Frontiers in Medicine. 9, 865722 (2022).
- Paredes, K., Capilla, J., Mayayo, E., Guarro, J. Virulence and experimental treatment of Trichoderma longibrachiatum, a fungus refractory to treatment. Antimicrobial Agents and Chemotherapy. 60 (8), 5029-5032 (2016).
- Perkhofer, S., Speth, C., Dierich, M. P., Lass-Flörl, C. In vitro determination of phagocytosis and intracellular killing of Aspergillus species by mononuclear phagocytes. Mycopathologia. 163 (6), 303-307 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved