A subscription to JoVE is required to view this content. Sign in or start your free trial.
Cryopreservation and Bioenergetic Evaluation of Human Peripheral Blood Mononuclear Cells
In This Article
Summary
Isolated peripheral blood mononuclear cells can be used for the analysis of immune functions and disorders, metabolic diseases, or mitochondrial functions. In this work, we describe a standardized method for the preparation of PBMCs from whole blood and the subsequent cryopreservation. Cryopreservation makes this time and place independent.
Abstract
The physiological functions of eukaryotic cells rely on energy mainly provided by mitochondria. Mitochondrial dysfunction is linked to metabolic diseases and aging. Oxidative phosphorylation plays a decisive role, as it is crucial for the maintenance of energetic homeostasis. PBMCs have been identified as a minimally invasive sample to measure mitochondrial function and have been shown to reflect disease conditions. However, measurement of mitochondrial bioenergetic function can be limited by several factors in human samples. Limitations are the amount of samples taken, sampling time, which is often spread over several days, and locations. Cryopreservation of the collected samples can ensure consistent collection and measurement of samples. Care should be taken to ensure that the parameters measured are comparable between cryopreserved and freshly prepared cells. Here, we describe methods for isolating and cryopreserving PBMCs from human blood samples to analyze the bioenergetic function of the mitochondria in these cells. PBMC cryopreserved according to the protocol described here show only minor differences in cell number and viability, adenosine triphosphate levels, and measured respiratory chain activity compared with freshly harvested cells. Only 8-24 mL of human blood is needed for the described preparations, making it possible to collect samples during clinical studies multicentrally and determine their bioenergetics on site.
Introduction
Human peripheral blood mononuclear cells (PBMCs) are used for various applications in many scientific fields, including the study of immunological and bioenergetic issues, such as those related to aging processes or degenerative diseases1,2. PBMCs are heterogeneous in composition and consist of lymphocytes (B cells, T cells, and NK cells), monocytes, and dendritic cells. The cells sometimes show great individual differences and variations within a subject, so standardized procedures for handling these cells are required. Important parameters such as viability and purity of the isolation are the basic requirements for its handling and are additionally influenced by environmental factors such as the time of collection, the melatonin level, whether the subject is fasting, and others3,4.
Based on studies on bioenergetics of PBMCs, we describe here a method for the isolation, cryopreservation, and cultivation of PBMCs that is suitable for other methods as well. While muscle biopsy is considered the gold standard for mitochondrial energy metabolism5, the examination of blood cells is a rapid, minimally invasive procedure. In addition to this, more and more studies suggest that the changes in mitochondrial function in aging and Alzheimer's disease (AD) occur not only in the brain but also in the periphery6,7,8,9,10. The method also allows investigations of other conditions and diseases, including diabetes mellitus and obesity11,12,13. Gene expression patterns in multiple sclerosis patients can be analyzed, or immune function and influences on it in general14,15,16.
PBMCs generally rely on oxidative phosphorylation (OXPHOS) to generate adenosine triphosphate (ATP)17,18. Therefore, PBMCs cover a wide range of applications as surrogates. In previous reports, the energy metabolism of PBMCs has been used to address organ dysfunctions, such as in early heart failure19, septic shock20 or sex-associated differences4 in mitochondrial function. A generalized method for cryopreservation isolation and cultivation of PBMCs would have advantages in the comparability of results obtained at different institutes. There is a great deal of variation in the protocols for each step21,22, the goal of this method is to provide a guideline for bioenergetic measurements in PBMCs.
In this article we describe a method for measuring bioenergetic parameters in PBMCs. We explain the methods for isolating, cryopreserving and measuring bioenergetics of PBMCs from human blood. This method can be used to determine bioenergetic parameters in patients and evaluate them in a clinical context. To apply these measurements, researchers need access to a patient population from which fresh blood samples can be obtained.
Protocol
All protocols described in this manuscript for blood collection, isolation and analysis have been reviewed and approved by the Institutional Review Board at the University of Giessen, Germany. The consent of the patients to include their samples in the study was obtained. All steps for isolation and cell culture are carried out under a biological safety cabinet.
1. Venipuncture
- Prepare all equipment needed for blood collection including disinfection spray, sterile swab, blood collection cannula with 80 mm tube and multi-adapter, tourniquet/blood pressure cuff, Monovette 9 mL lithium-heparin.
NOTE: EDTA as an anticoagulant is also effective. - Collect blood from the most appropriate arm vein, usually vena mediana cubiti or vena cephalica.
- Apply a tourniquet/blood pressure cuff with light pressure, around 80 mm/Hg.
- Disinfect gloves and puncture site with disinfectant spray containing alcohol. Allow the disinfected puncture site to air dry.
- The veins protrude due to the pressure of the pressure cuff. Insert the needle (Cannula diameter (outer) 21G / 0.8 mm, length 19 mm) at a 15°-20° angle of the vein attempting to avoid trauma and minimizing probing.
- Take blood with appropriate system, 4 tubes containing 9 mL of blood (more than 7-8 tubes are problematic for one experimenter to isolate properly).
- After blood collection, place the collection tubes in the dark for 5 min to ensure uniform anticoagulation.
2. PBMC isolation
- Prepare all needed solutions as described below.
- Bring Dulbecco's balanced salt solution (DPBS; concentration 1x) and lymphocyte isolation medium (1.077 g/mL) to room temperature (20-25 °C).
- Prepare Fetal bovine serum (FBS) at room temperature and keep one sterile 50 mL conical tube with FBS on ice. For each blood sample, 2 mL of FBS is required.
- Store freezing container at 4 °C and precool cryotubes at 4 °C.
- Warm cell culture medium to 37 °C, the medium consists of RPMI 1640 with 50 mL of FBS and penicillin 50 U/mL streptomycin 50 U/mL. This solution can be stored at 3 °C for up to 2 months.
- Add 8 mL of DPBS in sterile 50 mL conical tubes. Add 15 mL of lymphocyte isolation medium in sterile 50 mL conical tube (Medium is light sensitive, add it before starting isolation).
- Add 8 mL of blood to 8 mL of DPBS, and carefully further mix with a 3 mL plastic Pasteur pipette.
- Layer the blood/DPBS mix gently with a 3 mL plastic Pasteur pipette on top of the lymphocyte isolation medium. To apply the first layer to the medium, tilt the tube 20°-30°, which will result in less penetration of the blood-PBS mixture into the medium layer.
- Layer the blood-PBS mixture carefully over the side wall of the tube onto the lymphocyte isolation medium. Use a steady speed to keep the blood flow constant.
- In the next step, bring the tube slowly into an upright position, the remaining blood is carefully layered over the side wall of the tube onto the blood layer.
- Centrifuge for 10 min at 1000 x g at room temperature in a centrifuge with a swinging-bucket rotor with brakes off. After centrifugation, the blood/PBMC mix is separated into four layers. The top layer consists of plasma and platelets, the second layer is the PBMC layer, followed by a lymphocyte isolation medium layer, and, finally, erythrocytes and granulocytes in the bottom layer. The different layers are displayed in Figure 1.
- Remove 2/3rd of the plasma layer with a plastic Pasteur pipette.
- Using a 1 mL pipette, collect the PBMCs on the lymphocyte isolation medium layer taking care not to get any medium in the sample.
- Place the tip 1 mm above the PBMC layer. The PBMC layer should not be punctured, otherwise the medium will flow over the cells. The suction of the pipette pulls the PBMCs to this point so that they can be collected several times at this point.
NOTE: To maximize the number of PBMCs collected, at the end of the procedure search for the rest on the surface and try to collect the cells there as well. To stabilize the procedure, the tube can be placed on a surface. - Transfer the PBMCs step by step to a new 50 mL tube until the layer is completely harvested. Add DPBS up to the 25 mL mark, wash away lymphocyte isolation medium and other residues.
- Centrifuge for 10 min at 100 x g at room temperature with brakes on. Remove the supernatant with a vacuum pump or similar, take care not to damage the cell pellet.
- Resuspend the pellet in 1 mL of DPBS and add up DPBS to the 25 mL mark. Repeat washing once again and then resuspend in medium appropriate for the next steps.
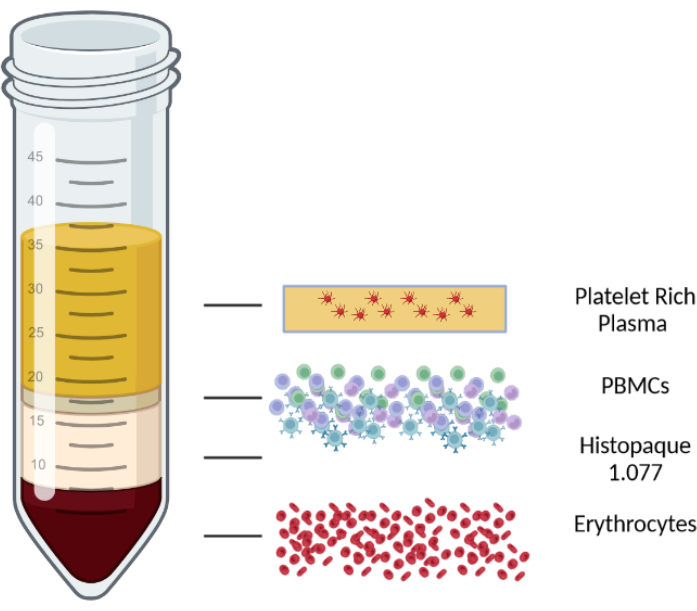
Figure 1: Schematic representation of a density gradient centrifugation to illustrate the different layers. Please click here to view a larger version of this figure.
3. Cryopreservation
- Cool down a freezing container to 4 °C, cool FBS on ice.
- Resuspend PBMC pellet in 1 mL of FBS with a 1 mL pipette, the FBS should be at room temperature.
- Mix DMSO with pre-cooled FBS on ice 1:5, final concentration 20% DMSO then put the FBS: DMSO mixture back on ice. Always prepare the solution fresh.
- Transfer the PBMC cell suspension in FBS to well labelled 2 mL cryotubes. Use one cryotube per collection tube. Adjust the number of cells between 1 x 107 and 5 x 107 per mL. Use an automated cell counter to determine cell count and viability.
- Add 1 mL of FBS: DMSO mixture dropwise with a 1 mL pipette, approximately 1-2 drops per s, into the tube. The dropwise addition leads to continuous and consistent mixing.
- Place tubes in the precooled freezing container. Place the freezing container in a -80 °C freezer for 24 h. The freezing container provides a controlled cooling of -1 °C per min.
- Remove the tubes from the -80 °C freezer. After removal from the freezer store the tubes in the gas phase of liquid nitrogen. Document the place of each sample.
4. Thawing
- Prepare all needed solutions: Cell culture medium RPMI 1640 with 50 mL of FBS and penicillin 50 U/mL, streptomycin 50 U/mL. This solution can be stored at 3°C for up to 2 months.
- Pre-warm cell culture medium to 37 °C. Add 3 mL of pre-warm cell culture medium into sterile 50 mL conical tubes.
- Remove sample from liquid nitrogen tank. Thaw samples in a water bath at 37 °C for approx. 3.5 min, remove from the water bath as soon as the last ice is melting. A pinhead-sized piece of ice should still be visible in the tube.
NOTE: DMSO is harmful to PBMCs work as quickly as possible. - Remove the cell:FBS:DMSO mix with a 1 mL pipette from the cryotube. Mix the PBMC samples with the cell culture medium in the prepared 50 mL tubes. Wash out the tubes with 5 mL of culture medium in three steps of 2 mL, 2 mL, and 1 mL.
- Transfer the medium into the tubes. This is performed to transfer possible cell residues. Centrifuge for 10 min at 100 x g at room temperature.
- Discard the supernatant and add 1 mL of medium appropriate for planned use. The cells are ready for subsequent experiments.
NOTE: However, with functional tests on fresh or frozen cells, a resting period in an incubator (typically overnight) is often recommended after lymphocyte isolation medium based isolation or cell thawing.
5. Cell culture
- After isolation or thawing culture cells overnight at 37 °C in 5% CO2/95% air.
- Resuspend the cells in 1 mL of RPMI medium supplemented with 10% FBS, penicilin 50 U/mL, streptomycin 50 U/mL. For further use there are many possibilities, treat cells as needed for the assays.
- For general storage use sterile 6 well cell culture plates and harvest cells after resting period. Transfer 1 mL of cell suspension with a 1 mL pipette into a well and add 4 mL of cell culture medium.
NOTE: The amount of PBMCs isolated varies greatly between individuals, one well with 5 mL of cell culture medium is sufficient for every 8 mL of blood isolated. However, when isolating PBMCs from buffy coats, the quantity of PBMCs is significantly higher than in whole blood samples and the cells should therefore be divided into several wells. - Let cells rest 24 h in a humidified atmosphere supplemented with 5% CO2 at 37 °C. This incubation is performed for freshly isolated cells, as well as for cryopreserved cells.
6. ATP assay
- Resuspend thawed PBMCs in 1 mL of RPMI medium supplemented with 10% FBS, penicillin 50 U/mL, streptomycin 50 U/mL.
- Take a sample and determine the cell count, then perform a live-dead discrimination with trypan blue. Take 10 µL from the resuspended cells and mix with 90 µL cell culture medium. Then take 10 µL and mix with 10 µL of trypan blue. Place the cells either in a cell counting chamber or an automatic cell counter and determine the number of live/dead cells.
- Plate 100 µL of cells at a density of 1 x 105 cells/100 µL per well in a 96-well white polystyrene plate.
- Let cells rest for 24 h in a humidified atmosphere supplemented with 5% CO2 at 37 °C.
- Determine the ATP concentrations with an ATP assay.
- Use light emission that occurs when ATP is combined with luciferin. The emitted light can be evaluated with a plate reader. Remove the plates for the incubator to cool down to room temperature for 15 min. Lyse the cells and leave them for 5 min. Then apply monitoring reagent to the cells and measure according to the manufacturer's instructions. An internal standard is used to determine the ATP level.
7. High-resolution respirometry
- Turn ON the high-resolution oxygraph and let it warm up for 30 min.
- Treat cells according to a protocol described in Figure 1. Prepare all stocks needed as in Table 1.
- Pipette 2.1 mL of respiration buffer (Table 1) into both high-resolution oxygraph chambers and stir the buffer continuously using a magnetic stirring bar present in the chambers (750 rpm) at 37 °C for 30 min until a stable oxygen flux signal of the polarographic oxygen sensor is obtained.
NOTE: In the chambers of the oxygraph, the oxygen consumption in real time (flux) and the oxygen saturation of the chamber are measured with the aid of polarographic oxygen electrodes. Background calibration must be performed to avoid background noise and to ensure reliable results. - Perform an air calibration of the polarographic oxygen sensor according to the manufacturer's protocols23.
- Resuspend isolated PBMCs in 1 mL of mitochondrial respiration medium (MIR05 the composition is shown in Table 1) and dilute to 8 x 106 cells/mL.
- After air calibration, aspirate the respiration medium from the oxygraph chamber and add 2.1 mL of cell suspension in each camber of the respirometer. If needed during the measurement reoxygenate the chambers (see Open at point h in Figure 2), the oxygen saturation of the chambers should not fall below 100 µM.
- Close the chambers by inserting the stoppers, the chambers are designed to hold 2.0 mL volume. Aspirate the emerging cell suspension.
- Continuously mix the cell suspension at 37 °C with a magnetic stirrer (750 rpm) located in the chamber. Wait for about 20 min until a stable signal is obtained. Determine endogenous respiration ((a) in Figure 2).
- To determine the different complex activities of the respiratory chain, inject the substrates and inhibitors for mitochondrial respiration through the titanium injection ports of the stoppers. Use the following final concentration in the chamber.
- To disrupt cell membranes, add 5 µL of 8.1 mM digitonin, through the titanium injection port of the chamber stopper, to remove naive substrates (b) in Figure 2 while the mitochondrial membranes remain intact.
- Add substrates 2 M glutamate and 800 mM malate through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min.
NOTE: It is also possible to use additional substrates like pyruvate. - Add 8 µL of 500 mM adenosine diphosphate (ADP) through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min (d) in Figure 2.
- Add 20 µL of 1 M succinate through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min (e) in Figure 2.
- Titrate 1 M carbonyl cyanide p-trifluoromethoxy phenylhydrazone (FCCP) stepwise at 0.5 µL up to the point where no further increase occurs. Wait 2-4 min until the signal stabilizes (f) in Figure 2. When there is no further increase in respiration continue with the next step.
CAUTION: Be careful when handling FCCP as it has health risks for humans. - Add 5 µL of 0.1 mM rotenone through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min (g) in Figure 2.
CAUTION: Be careful when handling rotenone as it has health risks for humans. - Add 1 µL of 4 mg/mL oligomycin through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min (h) in Figure 2.
CAUTION: Be careful when handling oligomycin as it is a poison that poses health risks to humans. - Add 1 µL of 5 mM antimycin A through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min (i) in Figure 2.
CAUTION: Be careful when handling antimycin A as it is a poison that poses health risks to humans. - When evaluating the run to exclude the oxygen consumption of enzymes not involved in oxidative phosphorylation, subtract the antimycin A values from all other measured values.
- Add 200 mM N,N,N',N'-tetramethyl-p-phenylenediamine dihydrochloride (TMPD; electron donator) and 800 mM ascorbate to keep TMPD in a reduced state. Inject the substrates through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min (j) in Figure 2.
- TMPD is subject to autooxidation, so subtract the resulting oxygen consumption from the measured value at Complex IV.
- Add NaN3 ≥ 100 mM through the injection port of the chamber stopper and record respiration until a stable signal is achieved. The signal stabilizes after 2-4 min to inhibit complex IV activity, only TMPD autooxidation remains.
CAUTION: Be careful when handling sodium azide as it is a poison that poses health risks to humans.

Figure 2: Schematic course of the O2 flux. The schematic course of the oxygen flux is shown. The curve is divided into the different phases after the addition of inhibitors and substrates from a-k. a: endogenous respiration; b: permeabilized cells; c: uncoupled complex I respiration; d: coupled complex I respiration; e: OXPHOS ; f: maximal uncoupled activity of CI and CII ; g: uncoupled respiration of complex II; h: leak respiration; i: residual respiration; j: CIV(U) uncoupled respiration and autooxidation of TMPD; k: autooxidation of TMPD. Please click here to view a larger version of this figure.
8. Citrate synthase activity
- Measure citrate synthase activity as a separate parameter and use it to normalize the measurements of the high-resolution oxygraph.
- Resuspend isolated PBMC in 1 mL of mitochondrial respiration medium (MIR05) and dilute to 8 x 106 cells/mL.
- Freeze in liquid nitrogen and store at −80 °C until experiments are conducted or to measure fresh cells.
- Prepare all solutions needed: 0.1 M triethanolamine HCl buffer pH 8.0, 1.0 M Tris-HCl buffer pH=8.1, 10% Triton X-100, 10 mM Oxalacetate in 0.1 M triethanolamine HCl buffer pH 8.0, 1.01 mM DTNB in 1.0 M Tris-HCl buffer pH=8.1, Acetyl-CoA 12.2 mM in double distilled H2O.
- Prepare reaction medium (Table 1) containing 5,5'-dithio-bis-(2-nitrobezoic acid) (DTNB; 0.1 mM), acetyl coenzyme A (0.31 mM), EDTA (50 µM), triethanolamine HCl (5 mM) and Tris HCl (0.1 M).
- Prepare starting reagent (Table 1) with 0.5 mM oxaloacetate dissolved in double distilled H2O.
- Thaw the samples on ice as citrate synthase is unstable if thawed too quickly.
- Add 40 µL of the samples to a 96-well plate on ice before adding 110 µL of reaction medium with a multipipette.
- Warm reaction medium and sample in an incubator to 30 °C for 5 min. Warm starting reagent in a water bath to 30 °C for 5 min.
- Add 50 µL of the starting reagent with a multipipette to each well. Measure absorbance at 30 °C at a wavelength of 412 nm for 20 min via plate reader.
Results
Cell viability and number
To achieve successful isolation and cryopreservation, cell count and viability should be as high as possible. Before and after cryopreservation, the cells are counted, and their viability is determined to ensure the health and quality of the cells. Figure 3 is a representative illustration of PBMCs before and after cryopreservation, cell count and viability hardly differ. This indicates successful isolation and preservation of PBMCs.
Discussion
This protocol provides a means of isolating and cryopreserving peripheral blood mononuclear cells (PBMCs) from human blood in a manner suitable for bioenergetic analyses. The described method offers the possibility to isolate PBMCs gently and in large quantities, with high viability and sufficient cells for bioenergetic measurements. It has the disadvantage that even with minimal interruptions, long isolations occur, but subsequent cryopreservation allows a time-independent measurement of bioenergetics. With this method,...
Disclosures
The authors declare no conflict of interest.
Acknowledgements
We would like to thank the clinical team of the University Hospital Giessen-Marburg for the blood collection. This work was funded by the Justus Liebig university.
Materials
| Name | Company | Catalog Number | Comments |
| 0.1 M Triethanolamine-HCl-Buffer (pH = 8,0) | Self-prepared | - | |
| 0.5 M Triethanolamine-HCl-Buffer | Self-prepared | - | |
| 1.0 M Tris-HCl-Buffer (pH = 8,1) | Self-prepared | - | |
| 1.01 mM DTBB | Self-prepared | - | |
| 10 % Triton X-100 | Self-prepared | - | |
| 10 mM Oxalacetat | Self-prepared | - | |
| 14–20 G sterile blood draw needles Multi Adapter Sarstedt Safety-Multifly | Sarstedt | 156353_v | |
| 37% HCl | Carl Roth GmbH & Co. KG | - | |
| 70% Ethanol (EtOH) | Self-prepared | - | |
| Acetyl-CoA | Pancreac Applichem | A3753 | |
| ADP | Sigma-Aldrich | A5285 | |
| Alcohol wipes | (70% isopropyl alcohol) | ||
| Antimycin A | Sigma-Aldrich | A8674 | |
| Aqua (bidest.) | With MilliQ Academic (self-made) | - | |
| Ascorbate | Sigma-Aldrich | A4034 | |
| ATP-Standard | Sigma-Aldrich | 6016949 | |
| Biocoll Seperating Solution | Biochrom | 6115 | |
| Biological safty cabinet MSC Advantage | Thermo Fisher Scientific Inc. | ||
| Carbonylcyanid-p-trifluoromethoxy-phenylhydrazon (FCCP) | Sigma-Aldrich | C2920 | |
| Cell counter TC20 Automated Cell Counter | Bio-Rad | ||
| Centrifuge Heraeus Megafuge 16 R | Thermo Fisher Scientific Inc. | ||
| Counting slides, dual chamber for cell counter | Bio-Rad | 1450016 | |
| Cryotube Cryo.S | Grainer Bio-One | 126263-2DG | |
| Digitonin | Sigma-Aldrich | 37008 | |
| Dimethylsulfoxid (DMSO) | Merck | 102952 | |
| Disinfection spray | |||
| Disposable gloves latex, rubber, or vinyl. | |||
| Distrips (12.5 ml) DistriTips | Gilson | F164150 | |
| Dulbecco’s Phosphate Buffered Saline (DPBS; 10x) | Gibco (Thermo Scientific) | 15217168 | |
| Ethanol (EtOH 100%) | Carl ROTH GmbH & Co. KG | 9065.3 | |
| Fetal bovine serum (FBS) | Sigma-Aldrich | F9665 | |
| Frezer (-80°C) | Thermo Fisher Scientific Inc. | ||
| Glutamate | Sigma-Aldrich | G1626 | |
| Holder/adapter | |||
| Incubator Midi 40 CO2 | Thermo Fisher Scientific Inc. | ||
| Injection syringe | Hamilton | ||
| Malate | Sigma-Aldrich | M-1000 | |
| MIR05 | Self-prepared | - | |
| Mr. Frosty Freezing Container | Thermo Fisher Scientific Inc. | 10110051 | |
| Multireader CLARIOstar | BMG Labtech | ||
| Nitrogen tank Locator 6 plus | Thermo Fisher Scientific Inc. | ||
| Oligomycin | Sigma-Aldrich | O4876 | |
| Oxalacetate | Sigma-Aldrich | - | |
| Oxygraph-2k | Orobororus Instruments | ||
| Penicillin-Streptomycin | PAA | 15140122 | |
| Pipettes Performance Pipettor 10 μL, 100 μL, 1000 μL | VWR | ||
| Roswell-Park. Memorial-Institute-Medium (RPMI-1640) | Gibco (Thermo Scientific) | 11530586 | |
| Rotenone | Sigma-Aldrich | R8875 | |
| Saccharose | Carl ROTH GmbH & Co. KG | 9286.2 | |
| Sodium azide | Sigma-Aldrich | S2002 | |
| Succinate | Sigma-Aldrich | S2378 | |
| Tetramethylphenylendiamin (TMPD) | Sigma-Aldrich | T3134 | |
| Tourniquet/ Blood pressure cuff | |||
| Tris(hydroxymethyl)amino-methane | Sigma-Aldrich | 108382 | |
| Triton X-100 | Sigma-Aldrich | 108643 | |
| Trypanblau | Biochrom | T6146 | |
| Vacuum pump | Vaccubrand GmbH & Co. | ||
| ViewPlate-96 | Perkin Elmer | 6005181 | |
| Water bath WNB22 | Memmert GmbH & Co. KG |
References
- Mancuso, M., et al. Mitochondria, cognitive impairment, and Alzheimer's disease. Int J Alzheimers Dis. 2009, 951548 (2009).
- Haas, R. H. Mitochondrial dysfunction in aging and diseases of aging. Biology. 8 (2), 48 (2019).
- Kleiveland, C. R., Verhoeckx, K., Cotter, P., Lopez-Exposito, I., et al. Peripheral blood mononuclear cells. The Impact of Food Bioactives on Health. In Vitro and Ex Vivo Models. , (2015).
- Silaidos, C., et al. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol Sex Differ. 9 (1), 34 (2018).
- Acin-Perez, R., Benincá, C., Shabane, B., Shirihai, O. S., Stiles, L. Utilization of human samples for assessment of mitochondrial bioenergetics: Gold standards, limitations, and future perspectives. Life. 11 (9), 949 (2021).
- Schindowski, K., et al. Impact of aging. NeuroMol Med. 4 (3), 161-177 (2003).
- Migliore, L., et al. Searching for the role and the most suitable biomarkers of oxidative stress in Alzheimer's disease and in other neurodegenerative diseases. Neurobiol Aging. 26 (5), 587-595 (2005).
- Leutz, S., et al. Reduction of trophic support enhances apoptosis in PC12 cells expressing Alzheimer’s APP mutation and sensitizes cells to staurosporine-induced cell death. J Mol Neurosci. 18 (3), 189-201 (2002).
- Leuner, K., et al. Peripheral mitochondrial dysfunction in Alzheimer’s disease: Focus on lymphocytes. Mol Neurobiol. 46 (1), 194-204 (2012).
- Leuner, K., et al. Enhanced apoptosis, oxidative stress and mitochondrial dysfunction in lymphocytes as potential biomarkers for Alzheimer’s disease. J Neural Transm Suppl. 2007 (72), 207-215 (2007).
- Kartika, R., Wibowo, H., Purnamasari, D., Pradipta, S., Larasati, R. A. Altered Indoleamine 2,3-Dioxygenase production and its association to inflammatory cytokines in peripheral blood mononuclear cells culture of type 2 diabetes mellitus. Int J Tryptophan Res. 13, 1178646920978236 (2020).
- Cortez-Espinosa, N., et al. CD39 expression on Treg and Th17 cells is associated with metabolic factors in patients with type 2 diabetes. Hum Immunol. 76 (9), 622-630 (2015).
- Mahmoud, F., et al. Effect of Diabetea tea ™ consumption on inflammatory cytokines and metabolic biomarkers in type 2 diabetes patients. J Ethnopharmacol. 194, 1069-1077 (2016).
- Volman, J. J., Ramakers, J. D., Plat, J. Dietary modulation of immune function by β-glucans. Physiol Behav. 94 (2), 276-284 (2008).
- Reddy, M., Eirikis, E., Davis, C., Davis, H. M., Prabhakar, U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 293 (1), 127-142 (2004).
- Otaegui, D., et al. Differential micro RNA expression in PBMC from multiple sclerosis patients. PLoS One. 4 (7), e6309 (2009).
- Geltink, R. I. K., Kyle, R. L., Pearce, E. L. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 36, 461-488 (2018).
- Fox, C. J., Hammerman, P. S., Thompson, C. B. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 5 (11), 844-852 (2005).
- Li, P., et al. Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci Rep. 5, 10229 (2015).
- Weiss, S. L., et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med. 16 (1), e4-e12 (2015).
- Higdon, L. E., Lee, K., Tang, Q., Maltzman, J. S. Virtual global transplant laboratory standard operating procedures for blood collection, PBMC isolation, and storage. Transplant Direct. 2 (9), e101 (2016).
- Betsou, F., Gaignaux, A., Ammerlaan, W., Norris, P. J., Stone, M. Biospecimen science of blood for peripheral blood mononuclear cell (PBMC) functional applications. Curr Pathobiol Rep. 7, 17-27 (2019).
- Pesta, D., Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 810, 25-58 (2012).
- Djafarzadeh, S., Jakob, S. M. High-resolution respirometry to assess mitochondrial function in permeabilized and intact cells. J Vis Exp. (120), e54985 (2017).
- Wang, W., Zhao, F., Ma, X., Perry, G., Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener. 15 (1), 30 (2020).
- Chaturvedi, R. K., Flint Beal, M. Mitochondrial diseases of the brain. Free Radic Biol Med. 63, 1-29 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved