A subscription to JoVE is required to view this content. Sign in or start your free trial.
Dual Test Gas Pulmonary Diffusing Capacity Measurement During Exercise in Humans Using the Single-Breath Method
In This Article
Summary
This protocol presents a method to assess pulmonary alveolar-capillary reserve measured by combined single-breath measurement of the diffusing capacity to carbon monoxide (DL,CO) and nitric oxide (DL,NO) during exercise. Assumptions and recommendations for using the technique during exercise form the foundation of this article.
Abstract
The combined single-breath measurement of the diffusing capacity of carbon monoxide (DL,CO) and nitric oxide (DL,NO) is a useful technique to measure pulmonary alveolar-capillary reserve in both healthy and patient populations. The measurement provides an estimate of the participant's ability to recruit and distend pulmonary capillaries. The method has recently been reported to exhibit a high test-retest reliability in healthy volunteers during exercise of light to moderate intensity. Of note, this technique permits up to 12 repeated maneuvers and only requires a single breath with a relatively short breath-hold time of 5 s. Representative data are provided showing the gradual changes in DL,NO and DL,CO from rest to exercise at increasing intensities of up to 60% of maximal workload. The measurement of diffusing capacity and evaluation of alveolar-capillary reserve is a useful tool to evaluate the lung's ability to respond to exercise both in the healthy population as well as in patient populations such as those with chronic lung disease.
Introduction
Exercise leads to a considerable increase in energy demand compared to the resting state. The heart and lungs respond by increasing cardiac output and ventilation resulting in an expansion of the alveolar-capillary bed, mainly the recruitment and distention of pulmonary capillaries1. This ensures a sufficient pulmonary gas exchange, which can be measured by an increase in pulmonary diffusing capacity (DL)2,3,4. The first attempts to measure DL during exercise date back more than a century5,6,7. The ability to increase DL from the resting state is often referred to as the alveolar-capillary reserve8, 9.
Experimentally, the relative contributions of alveolar-capillary membrane diffusing capacity (DM) and pulmonary capillary blood volume (VC) to alveolar-capillary reserve can be assessed by different methods, including the classical multiple fractions of inspired oxygen ( ) method10. An alternative technique that may be useful in this context is the dual-test gas method, in which the DL to carbon monoxide (CO) and nitric oxide (NO) (DL,CO/NO) are concurrently measured11. This technique was developed in the 1980s, and takes advantage of the fact that the reaction rate of NO with hemoglobin (Hb) is substantially greater than that of CO, such that the pulmonary diffusion of CO depends more on VC than does NO. Hence, the main site of resistance (~75%) to CO diffusion is located within the red blood cell, while the main resistance (~60%) to NO diffusion is at the alveolar-capillary membrane and pulmonary plasma12. The concurrent measurement of DL,CO and DL,NO thus permits the assessment of the relative contributions of DM and VC to DL12, where the change in DL,NO observed during exercise thus largely reflects the expansion of the alveolar-capillary membrane. An additional advantage of this method when obtaining measurements during exercise is that it involves a relatively short breath-hold time (~5 s) and fewer maneuvers compared to the classical
) method10. An alternative technique that may be useful in this context is the dual-test gas method, in which the DL to carbon monoxide (CO) and nitric oxide (NO) (DL,CO/NO) are concurrently measured11. This technique was developed in the 1980s, and takes advantage of the fact that the reaction rate of NO with hemoglobin (Hb) is substantially greater than that of CO, such that the pulmonary diffusion of CO depends more on VC than does NO. Hence, the main site of resistance (~75%) to CO diffusion is located within the red blood cell, while the main resistance (~60%) to NO diffusion is at the alveolar-capillary membrane and pulmonary plasma12. The concurrent measurement of DL,CO and DL,NO thus permits the assessment of the relative contributions of DM and VC to DL12, where the change in DL,NO observed during exercise thus largely reflects the expansion of the alveolar-capillary membrane. An additional advantage of this method when obtaining measurements during exercise is that it involves a relatively short breath-hold time (~5 s) and fewer maneuvers compared to the classical  technique, where multiple repeated maneuvers with a standardized 10 s breath-hold are performed at different oxygen levels. Although
technique, where multiple repeated maneuvers with a standardized 10 s breath-hold are performed at different oxygen levels. Although  has recently been applied with a shorter breath-hold time and fewer maneuvers at each intensity13. Nevertheless,
has recently been applied with a shorter breath-hold time and fewer maneuvers at each intensity13. Nevertheless,  only permits a total of six DL,CO maneuvers per session, whereas up to 12 repeated DL,CO/NO maneuvers can be performed without any measurable effect on the resultant estimates14. These are important considerations when obtaining measurements during exercise since both a long breath-hold and multiple maneuvers may be difficult to perform at very high intensities or in patient populations who experience dyspnea.
only permits a total of six DL,CO maneuvers per session, whereas up to 12 repeated DL,CO/NO maneuvers can be performed without any measurable effect on the resultant estimates14. These are important considerations when obtaining measurements during exercise since both a long breath-hold and multiple maneuvers may be difficult to perform at very high intensities or in patient populations who experience dyspnea.
The present paper provides a detailed protocol, including theoretical considerations and practical recommendations on the measurement of DL,CO/NO during exercise and its use as an index of the alveolar-capillary reserve. This method is easily applicable in the experimental setting and permits the assessment of how diffusion limitation in the lungs may affect oxygen uptake in different populations.
Theory and measurement principles
The DL,CO/NO method involves a single breath of a gas mixture with the assumption that the gases distribute equally in the ventilated alveolar space after inhalation. The gas mixture consists of several gases including an inert tracer gas. The dilution of the tracer gas in the ventilated alveolar space, as based on its fraction in end-expiratory air, can be used to calculate the alveolar volume (VA)15. The gas mixture also includes the test gas CO and NO, both of which are diluted in the ventilated alveolar space and diffuse across the alveolar-capillary membrane. Based on their alveolar fractions, their individual rates of disappearance (k), also termed the diffusing constant, from the alveolar space can be calculated. By convention, the DL for a test gas measured during a single-breath maneuver, is derived by the following equation16:
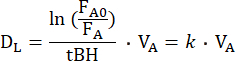
where FA0 is the alveolar fraction of the test gas (CO or NO) at the onset of the breath-hold of the individual DL maneuver, while FA is the alveolar fraction of the test gas at the end of the breath-hold, and tBH is the breath-hold time. DL is mechanically equivalent to the conductance of the test gas across the alveolar-capillary membrane, through plasma and the red blood cell interior to hemoglobin. It thus depends both on the conductance of DM and the so-called specific conductance of pulmonary capillary blood (θ), of which the latter depends both on the conductance of the test gas in blood and on its reaction rate with hemoglobin10. Given that the reciprocal of conductance is resistance, the total resistance to the transfer of a test gas depends on the following resistances in series10:
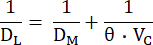
These components may be distinguished by concurrently measuring the DL to CO and NO, because these have different θ-values, and their respective DL values thus depend differently on VC. The pulmonary diffusion of CO depends more heavily on VC than does NO, with the main site of resistance (~75%) to CO diffusion being located within the red blood cell12. In contrast, the main resistance (~60%) to NO diffusion is at the alveolar-capillary membrane and pulmonary plasma, because the reaction rate of NO with hemoglobin is substantially greater than that of CO. Hence, by concurrently measuring DL,CO and DL,NO, changes in both DM and VC will markedly affect the former, while the latter will depend much less on VC, thus permitting an integrative assessment of the factors that determine DL.
The reporting of DL,CO/NO metrics may be done using different units. Hence, the European respiratory society (ERS) uses mmol/min/kPa, whereas the American Thoracic society (ATS) uses mL/min/mmHg. The conversion factor between the units is 2.987 mmol/min/kPa = mL/min/mmHg.
Protocol
The Scientific Ethical Committee for the Capital Region of Denmark has previously approved the measurement of DL,CO/NO at rest, during exercise, and in the supine position in both healthy volunteers and patients with chronic obstructive pulmonary disease (COPD) at our institution (protocols H-20052659, H-21021723, and H-21060230).
NOTE: Before DL,CO/NO is measured during exercise, a dynamic spirometry, and a cardiopulmonary exercise test (CPET) must be performed. The dynamic spirometry is used for quality control of the individual DL,CO/NO maneuvers, while the CPET is used to determine the workload at which DL,CO/NO is to be measured during exercise. In patients with airflow limitation, notably due to obstructive lung disease, it may be advantageous to supplement the dynamic spirometry with a whole body-plethysmography to obtain a valid measure of vital capacity. A medical health check to rule out any known contraindications before initiating CPET is recommended17. Importantly, the CPET should be performed at least 48 h prior to the DL,CO/NO measurement obtained during exercise, as prior vigorous exercise may affect DL for up to at least 24 h18,19.
1. Dynamic spirometry
NOTE: Dynamic spirometry should be performed in accordance with the current clinical guidelines from the ERS and ATS20.
- Measure weight (to the nearest 100 g) and height (to the nearest 1 mm).
- Ask the participant to sit in an upright chair.
- Perform a dynamic spirometry during a forced expired maneuver to identify the forced expired volume in 1 s (FEV1) and forced vital capacity (FVC) of the participant, as described elsewhere20.
2. Cardiopulmonary exercise test (CPET)
NOTE: CPET should be performed in alignment with current clinical recommendations21.
- Adjust the cycle ergometer according to the participant's height and place a heart rate (HR) monitor on the chest.
- Place the participant on the cycle ergometer. Equip the participant with a mask connected to a metabolic measurement system, to measure ventilation and pulmonary gas exchange throughout the test.
- Instruct the participant to begin cycling at a self-selected pace ≥60 rounds per min (RPM) and perform a 5 min warm-up period at a submaximal workload based on self-reported activity level, daily fitness and disease status (e.g., 15-150 W).
- Increase the workload by 5-20 W every min until the participant reaches voluntary exhaustion. The increments should be based on the participant's current fitness level, so that the test is expected to terminate 8-12 min after the commencement of the incremental phase.
- Instruct the participant to avoid other vigorous exercise for the next 48 h.
3. Calibration of single breath diffusing capacity equipment
NOTE: It is necessary to calibrate flow sensors and gas analyzers to ensure that measurements are both valid and reliable. The exact procedure is manufacturer- and device-specific. The calibration procedure, including biological control, should be completed on each study day, and if less than one study day is executed per week, additional weekly calibrations should be performed. The experimental setup is shown in Figure 1.
- Open the software program on the computer, and an automatic warmup period of 50 min will be initiated to ensure sufficient temperature of the pneumotach.
- Make sure that the containers with the test gases are open (See Figure 1D).
- Perform a gas calibration by first connecting the sampling line from the pneumotach to the MS-PFT Analyzer Unit plug-in termed CAL (See Figure 1B).
- Initiate the gas calibration by selecting Calibration on the Home Page (See Figure 2A) and choose Gas calibration. Start the calibration by pressing Start or F1 (See Figure 2B).
- Attach the sampling line to the pneumotach when the gas calibration is fulfilled and accepted.
- Perform a volume calibration using a valid 3 L syringe. Initiate the volume calibration by selecting Calibration on the Home Page (See Figure 2A) and choose Volume calibration. Start the calibration by pressing F1, and follow the instruction provided by the software (See Figure 2C).
- Make sure that the inspiratory bag is connected to MS-PFT analyzer unit (See Figure 1C).
- Complete the calibration procedure by performing a biological control measurement at rest in the sitting position. This should be performed by a healthy non-smoker to ensure reliability of the method. If the given subject's week-to-week variation in DL,CO or DL,NO varies more than 1.6 and 6.5 mmol/min/kPa (5 and 20 mL/min/mmHg), respectively, the variation can be due to machine error and should be investigated further12, 22.
4. Preparation of the participant
- Calculate the desired workload from the prior CPET results for the chosen intensity (% of maximal workload (Wmax)) at which the DL,CO/NO will be measured.
- At least 48 h after the participant has performed the CPET, ask the participant to return to the laboratory to obtain the DL,CO/NO measurement during exercise.
- Measure the height (in cm to the nearest mm), weight (in kg to the nearest 100 g) and Hb from capillary blood (in mmol/L to the nearest 0.1 mmol/L) of the patient.
- On the Home Page of the program choose Patient > New patient (See Figure 2A) and fill in the required data: Identification, Last Name, First Name, Date of Birth, Gender, Height, and Weight of the participant. Continue by selecting OK or F1 (See Figure 2D).
5. DL,CO/NO measurement during upright rest
NOTE: DL,CO/NO measurements are performed in accordance with current clinical recommendations from ERS task force12.
- On the Home Page choose Measurement > NO Membrane diffusing (See Figure 2E).
- Start the automatic resetting of the software, to zero the gas analyzer for all tests gases and to initiate the mixing of the test gases in the connected inspiratory bag. Initiate the automatic resetting by pressing F1 (See Figure 2F).
- The automatic resetting takes 140-210 s. Observe the instructions provided by the software to recognize when to initiate the measurement. It is important to initiate the measurement immediately when the software instructs to Connect patient.
- Place the participant in an upright chair equipped with a nose-clip. Instruct the participant on how to perform the maneuver as described below.
- Ask the participant to use the nose-clip and begin normal tidal respirations through a mouthpiece connected to the pneumotach. To ensure a closed system for the measurements, make sure that the participant's lips are kept closed around the mouthpiece.
- After three normal respirations, instruct the participant to perform a rapid maximal expiration to reach residual volume (RV).
- When RV is reached, immediately instruct the participant to perform a rapid maximal inspiration to total lung capacity (TLC), targeting an inspiratory time of < 4 s. During the maximal inspiration, a valve opens, allowing the participant to inhale the gas mixture mixed with a known concentration of NO (800 ppm NO/N2) in an inspiratory bag just prior to the inhalation.
- Ask the participant to carry out a breath-hold of 5 (4-8) s at TLC. During the inspiration an inspired volume (VI) ≥90% of the FVC (or plethysmography-based vital capacity) with a 4-8 s breath-hold time is targeted23 (Table 1).
- After the breath-hold, instruct the participant to perform a strong steady maximal expiration with no interruptions.
- After the maximal expiration ask the participant to let go of the mouthpiece and nose-clip. The software will then calculate DL,NO and DL,CO without any command.
- Use verbal encouragement throughout the maneuver to ensure that the participant reaches RV and TLC. Assess the acceptability of the maneuver as per Table 1.
- Perform the maneuver again after at least a 4 min wash out period, and until two maneuvers fulfil the acceptability criteria (Table 1) or until a total of 12 maneuvers (see below) have been performed on the same session.
- The DL,NO and DL,CO are reported according to the criteria outlined in Table 2. We also recommend that breath-hold time, inspired volume, and alveolar volume as reported. Furthermore, the number of acceptable and repeatable maneuvers should be reported, and findings based on maneuvers that either do not fulfill the acceptability or repeatability criteria should be interpreted with caution.
6. DL,CO/NO measurement during exercise
NOTE: A timeline of DL,CO/NO measurements during exercise is provided in Figure 3.
- Place the cycle ergometer at a distance that enables the participant to breathe through the mouthpiece without having to change the cycling position. Increase the height of the equipment so that the measurements can be carried out with a correct working position on the bicycle (See Figure 2).
- Place the participant on the cycle ergometer and place a HR monitor on the chest. Instruct the participant to perform each maneuver as outlined in step 5.3.
- Instruct the participant to begin cycling for 5 min at a submaximal workload, as a warm-up prior to the measurement.
- Increase the workload to the target intensity while simultaneously starting the automatic resetting of the device by pressing F1 (see step 5.2). The automatic resetting takes 140-210 s, which is sufficient to ensure that the participant has reached steady state.
- When the automatic resetting is finished, turn the mouthpiece to the participant and perform a maneuver as described below while the participant continues cycling at the target intensity.
- Follow the steps in steps 5.4 to 5.5. Assess acceptability and repeatability criteria (Table 1) at each workload, and report as for measurements during rest (see step 5.6 and Table 2).
- After completion of the maneuver, remove the mouthpiece and decrease the workload to 15-40 W. Perform the active recovery phase for 2 min after which repeat steps 6.4 and 6.5. The 2 min of active recovery and the 140-210 s during the automatic resetting provides a sufficient washout period of 4-5 min.
Results
The protocol was implemented in 2021 and at the time of writing a total of 124 measurements during exercise (i.e., 51 in healthy volunteers and 73 in patients with COPD of various severities) had been performed. The maneuvers, as well as data on fulfilled acceptability and repeatability criteria, and the failure rate are all provided in Table 3.
Calculations
As an example, calculations from a single DL,CO/NO maneuver are ...
Discussion
The protocol provides a standardized approach to the measurement of DL,CO/NO during exercise using the dual test gas single-breath technique. Since the obtained DL,CO/NO-metrics increase due to pulmonary capillary recruitment and distension, the method provides a physiologically meaningful measure of the alveolar-capillary reserve.
Critical steps in the protocol
The method requires an exhalation to residual volume followed by an inspiration to total...
Disclosures
The equipment and software presented in the article is not free of charge. None of the authors is associated with any company providing the license to the software. All authors declare no competing financial interests.
Acknowledgements
The study received financial support from The Svend Andersen Foundation. The Centre for Physical Activity Research is supported by TrygFonden Grants ID 101390, ID 20045, and ID 125132. JPH is funded by HelseFonden and Copenhagen University Hospital, Rigshospitalet, while HLH is funded by the Beckett Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| HemoCue Hb 201+ | HemoCue, Brønshøj, Denmark | Unkown | For measurements of hemoglobin |
| Jaeger MasterScreen PFT pro (Lung Function Equipment) | CareFusion, Höchberg, Germany | Unkown | For measurements of DLCO/NO |
| Mouthpiece | SpiroBac, Henrotech, Aartselaar, Belgium | Unkown | Used together with the Lung Fuction Equipment. (dead space 56 ml, resistance to flow at 12 L s−1 0.9 cmH2O) |
| Nose-clip | IntraMedic, Gentofte, Denmark | JAE-892895 | |
| Phenumotach | IntraMedic, Gentofte, Denmark | JAE-705048 | Used together with the Lung Fuction Equipment |
| SentrySuite Software Solution | Vyaire's Medical GmbH, Leibnizstr. 7, D-97204 Hoechberg Germany | Unkown | |
| Test gasses | IntraMedic, Gentofte, Denmark | Unkown | Concentrations: 0.28% CO, 20.9% O2, 69.52% N2 and 9.3% He |
References
- Johnson Jr, R. L., Heigenhauser, G. J. F., Hsia, C. C., Jones, N. L., Wagner, P. D. Determinants of gas exchange and acid-base balance during exercise. Compr Physiol. , 515-584 (2011).
- Rampulla, C., Marconi, C., Beulcke, G., Amaducci, S. Correlations between lung-transfer factor, ventilation, and cardiac output during exercise. Respiration. 33 (6), 405-415 (1976).
- Tedjasaputra, V., Bouwsema, M. M., Stickland, M. K. Effect of aerobic fitness on capillary blood volume and diffusing membrane capacity responses to exercise. J Physiol. 594 (15), 4359-4370 (2016).
- Tamhane, R. M., Johnson, R. L., Hsia, C. C. W. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 120 (6), 1850-1856 (2001).
- Bohr, C. On the determination of gas diffusion through the lungs and its size during rest and work. Zentralblatt für Physiologie. 23 (12), 374-379 (1909).
- Krogh, A., Krogh, M. On the rate of diffusion of carbonic oxide into the lungs of man. Skandinavisches Archiv Für Physiologie. 23 (1), 236-247 (1910).
- Krogh, M. The diffusion of gases through the lungs of man. J Physiol. 49 (4), 271-300 (1915).
- Hsia, C. C., Herazo, L. F., Ramanathan, M., Johnson, R. L. Cardiopulmonary adaptations to pneumonectomy in dogs IV. Membrane diffusing capacity and capillary blood volume. J Appl Physiol. 77 (2), 998-1005 (1994).
- Behnia, M., Wheatley, C. M., Avolio, A., Johnson, B. D. Alveolar-capillary reserve during exercise in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 12, 3115-3122 (2017).
- Roughton, F. J., Forster, R. E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 11 (2), 290-302 (1957).
- Borland, C., Higenbottam, T. A simultaneous single breath measurement of pulmonary diffusing capacity with nitric oxide and carbon monoxide. Eur Respir J. 2 (1), 56-63 (1989).
- Zavorsky, G. S., et al. Standardisation and application of the single-breath determination of nitric oxide uptake in the lung. Eur Respir J. 49 (2), 1600962 (2017).
- Tedjasaputra, V., Van Diepen, S., Collins, S., Michaelchuk, W. M., Stickland, M. K. Assessment of pulmonary capillary blood volume, membrane diffusing capacity, and intrapulmonary arteriovenous anastomoses during exercise. J Vis Exp. (120), e54949 (2017).
- Zavorsky, G. S. The rise in carboxyhemoglobin from repeated pulmonary diffusing capacity tests. Respir Physiol Neurobiol. 186 (1), 103-108 (2013).
- Graham, B. L., et al. ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 49 (1), 1600016 (2017).
- Hughes, J. M., Pride, N. B. Examination of the carbon monoxide diffusing capacity (DLCO) in relation to its KCO and VA components. Am J Respir Crit Care Med. 186 (2), 132-139 (2012).
- Balady, G. J., et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American heart association. Circulation. 122 (2), 191-225 (2010).
- Hanel, B., Clifford, P. S., Secher, N. H. Restricted postexercise pulmonary diffusion capacity does not impair maximal transport for O2. J Appl Physiol. 77 (5), 2408-2412 (1994).
- Sheel, A. W., Coutts, K. D., Potts, J. E., McKenzie, D. C. The time course of pulmonary diffusing capacity for carbon monoxide following short duration high intensity exercise. Respir Physiol. 111 (3), 271-281 (1998).
- Graham, B. L., et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 200 (8), e70-e88 (2019).
- Glaab, T., Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir Res. 23 (1), 9 (2022).
- Munkholm, M., et al. Reference equations for pulmonary diffusing capacity of carbon monoxide and nitric oxide in adult Caucasians. Eur Respir J. 52 (1), 1500677 (2018).
- Dressel, H., et al. Lung diffusing capacity for nitric oxide and carbon monoxide: dependence on breath-hold time. Chest. 133 (5), 1149-1154 (2008).
- Madsen, A. C., et al. Pulmonary diffusing capacity to nitric oxide and carbon monoxide during exercise and in the supine position: a test-retest reliability study. Exp Physiol. 108 (2), 307-317 (2023).
- Ross, B. A., et al. The supine position improves but does not normalize the blunted pulmonary capillary blood volume response to exercise in mild COPD. J Appl Physiol. 128 (4), 925-933 (2020).
- Zavorsky, G. S., Lands, L. C. Lung diffusion capacity for nitric oxide and carbon monoxide is impaired similarly following short-term graded exercise. Nitric Oxide. 12 (1), 31-38 (2005).
- Alves, M. M., Dressel, H., Radtke, T. Test-retest reliability of lung diffusing capacity for nitric oxide during light to moderate intensity cycling exercise. Respir Physiol Neurobiol. 304, 103940 (2022).
- Jorgenson, C. C., Coffman, K. E., Johnson, B. D. Effects of intrathoracic pressure, inhalation time, and breath hold time on lung diffusing capacity. Respir Physiol Neurobiol. 258, 69-75 (2018).
- Zavorsky, G. S., Quiron, K. B., Massarelli, P. S., Lands, L. C. The relationship between single-breath diffusion capacity of the lung for nitric oxide and carbon monoxide during various exercise intensities. Chest. 125 (3), 1019-1027 (2004).
- Coffman, K. E., Boeker, M. G., Carlson, A. R., Johnson, B. D. Age-dependent effects of thoracic and capillary blood volume distribution on pulmonary artery pressure and lung diffusing capacity. Physiol Rep. 6 (17), e13834 (2018).
- Borland, C. D. R., Hughes, J. M. B. Lung diffusing capacities (DL) for nitric oxide (NO) and carbon monoxide (CO): The evolving story. Compr Physiol. 11 (1), 1371 (2021).
- Tedjasaputra, V., Van Diepen, S., Collins, S. &. #. 2. 0. 1. ;., Michaelchuk, W. M., Stickland, M. K. Assessment of pulmonary capillary blood volume, membrane diffusing capacity, and intrapulmonary arteriovenoua anastomoses during exercise. J. Vis. Exp. (120), e54949 (2017).
- Thomas, A., et al. The single-breath diffusing capacity of CO and NO in healthy children of European descent. PLoS One. 12 (6), e0179097 (2017).
- Blakemore, W. S., Forster, R. E., Morton, J. W., Ogilvie, C. M. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest. 36 (1), 1-17 (1957).
- Cotes, J. E., et al. Iron-deficiency anaemia: its effect on transfer factor for the lung (diffusiong capacity) and ventilation and cardiac frequency during sub-maximal exercise. Clin Sci. 42 (3), 325-335 (1972).
- Mann, T., Lamberts, R. P., Lambert, M. I. Methods of prescribing relative exercise intensity: Physiological and practical considerations. Sports Med. 43 (7), 613-625 (2013).
- Forster, R. E. Exchange of gases between alveolar air and pulmonary capillary blood: pulmonary diffusing capacity. Physiol Rev. 37 (4), 391-452 (1957).
- Tedjasaputra, V., et al. Pulmonary capillary blood volume response to exercise is diminished in mild chronic obstructive pulmonary disease. Respir Med. 145, 57-65 (2018).
- Nymand, S. B., et al. Exercise adaptations in COPD: the pulmonary perspective. Am J Physiol Lung Cell Mol Physiol. 323 (6), L659-L666 (2022).
- Rodríguez-Roisin, R., et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol. 106 (6), 1902-1908 (2009).
- Hsia, C. C., Johnson, R. L., Shah, D. Red cell distribution and the recruitment of pulmonary diffusing capacity. J Appl Physiol. 86 (5), 1460-1467 (1999).
- Wilhelm, E., Battino, R., Wilcock, R. J. Low-pressure solubility of gases in liquid water. Chem Rev. 77 (2), 219-262 (1977).
- Forster, R. E. . Diffusion of gases across the alveolar membrane. , (1987).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved