A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Cost-Efficient Transcriptomic-Based Drug Screening
In This Article
Summary
This protocol describes a workflow from ex vivo or in vitro cell cultures to transcriptomic data pre-processing for cost-effective transcriptome-based drug screening.
Abstract
Transcriptomics allows to obtain comprehensive insights into cellular programs and their responses to perturbations. Despite a significant decrease in the costs of library production and sequencing in the last decade, applying these technologies at the scale necessary for drug screening remains prohibitively expensive, obstructing the immense potential of these methods. Our study presents a cost-effective system for transcriptome-based drug screening, combining miniaturized perturbation cultures with mini-bulk transcriptomics. The optimized mini-bulk protocol provides informative biological signals at cost-effective sequencing depth, enabling extensive screening of known drugs and new molecules. Depending on the chosen treatment and incubation time, this protocol will result in sequencing libraries within approximately 2 days. Due to several stopping points within this protocol, the library preparation, as well as the sequencing, can be performed time-independently. Processing simultaneously a high number of samples is possible; measurement of up to 384 samples was tested without loss of data quality. There are also no known limitations to the number of conditions and/or drugs, despite considering variability in optimal drug incubation times.
Introduction
The development of new drugs is a complex and time-consuming process that involves identifying potential drugs and their targets, optimizing and synthesizing drug candidates, and testing their efficacy and safety in preclinical and clinical trials1. Traditional methods for drug screening, i.e., the systematic assessment of libraries of candidate compounds for therapeutic purposes, involve the use of animal models or cell-based assays to test the effects on specific targets or pathways. While these methods have been successful in identifying drug candidates, they often did not provide sufficient insights into the complex molecular mechanisms underlying drug efficacy and also toxicity and mechanisms of potential side effects.
Assessing genome-wide transcriptional states presents a powerful approach to overcome current limitations in drug screening, as it enables comprehensive assessments of gene expression in response to drug treatments2. By measuring RNA transcripts in a genome-wide fashion expressed at a given time, transcriptomics aims to provide a holistic view of the transcriptional changes that occur in response to drugs, including changes in gene expression patterns, alternative splicing, and non-coding RNA expression3. This information can be used to determine drug targets, predict drug efficacy and toxicity, and optimize drug dosing and treatment regimens.
One of the key benefits of combining transcriptomics with unbiased drug screening is the potential to identify new drug targets that have not been previously considered. Conventional drug screening approaches often focus on established target molecules or pathways, hindering the identification of new targets and potentially resulting in drugs with unforeseen side effects and restricted effectiveness. Transcriptomics can overcome these limitations by providing insights into the molecular changes that occur in response to drug treatment, uncovering potential targets or pathways that may not have been previously considered2.
In addition to the identification of new drug targets, transcriptomics can also be used to predict drug efficacy and toxicity. By analyzing the gene expression patterns associated with drug responses, biomarkers can be developed that can be used to predict a patient's response to a particular drug or treatment regimen. This can also help to optimize drug dosing and reduce the risk of adverse side effects4.
Despite its potential benefits, the cost of transcriptomics remains a significant barrier to its widespread application in drug screening. Transcriptomic analysis requires specialized equipment, technical expertise, and data analysis, which can make it challenging for smaller research teams or organizations with limited funding to utilize transcriptomics in drug screening. However, the cost of transcriptomics has been steadily decreasing, making it more accessible to the research communities. Additionally, advancements in technology and data analysis methods have made transcriptomics more efficient and cost-effective, further increasing its accessibility2.
In this protocol, we describe a high-dimensional and explorative system for transcriptome-based drug screening, combining miniaturized perturbation cultures with mini-bulk transcriptomics analysis5,6. With this protocol, it is possible to reduce the cost per sample to 1/6th of the current cost of commercial solutions for full-length mRNA sequencing. The protocol requires only standard laboratory equipment, the only exception being the use of short-read sequencing technologies, which can be outsourced if sequencing instruments are not available in-house. The optimized mini-bulk protocol provides information-rich biological signals at cost-effective sequencing depth, enabling extensive screening of known drugs and new molecules.
The aim of the experiment is to screen for drug activity on PBMCs in different biological contexts. This protocol can be applied to any biological question where several drugs should be tested with a transcriptomic readout, giving a transcriptome-wide view of the cellular effect of the treatment.
Protocol
This protocol follows the guidelines of the local ethics committees of the University of Bonn.
1. Preparation of buffers, solutions, and equipment
- Prepare the solutions and gather the materials described in Table of Materials.
- Heat up the water bath to 37 °C and warm up the complete growth medium (RPMI-1640 + 10% fetal calf serum (FCS) + 1% penicillin/streptomycin).
- For cell harvesting, use ice-cold phosphate-buffered saline (PBS).
NOTE: Keep a clean environment while working with cells to maintain sterility.
2. Cell handling
NOTE: A detailed protocol for the cryopreservation of peripheral blood mononuclear cells (PBMC) from human blood can be found in7.
- Cell thawing and counting
- Remove the cryovials from liquid nitrogen and thaw them in a water bath at 37 °C for 2 - 3 min while gently inverting.
- Transfer the thawed cells into a 50 mL conical tube.
- Rinse the cryovial with 1 mL warm complete growth medium and add this solution dropwise to the cells in the tube (1st dilution step).
- Repeat the dilution 1:1 until reaching a volume of 32 mL (5 dilutions total with respectively 1, 2, 4, 8, and 16 mL of warm complete growth medium). Add the medium dropwise to reduce cell disturbance while slightly agitating the conical tube.
- Centrifuge the cell suspension for 5 min (300 x g, 20 °C) and remove the supernatant by gently tipping the conical tube in one fluent motion.
- Resuspend the cells in 3 mL of warm complete growth medium and proceed with cell counting.
- For counting, mix 10 µL of cell suspension with Trypan Blue (1:2 to 1:10 dilution depending on the density of the cell suspension) to distinguish living from dead cells while counting. Dead or damaged cells will appear blue due to the uptake of the dye, while vital cells are not stained.
NOTE: Trypan Blue is slightly cytotoxic, stained cells should not be stored for more than 5 min. - Count the cells with either an automated cell counter or counting chamber by using 10 µL of stained cell solution.
- When using the Neubauer-improved cell chamber, count all cells within the four large squares located at the corners and use the following equation to calculate the concentration of the cell suspension.
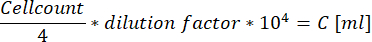
- When using the Neubauer-improved cell chamber, count all cells within the four large squares located at the corners and use the following equation to calculate the concentration of the cell suspension.
- Dilute the cells to a final concentration of 1 x 106 cells/mL with warm complete growth medium. Centrifuge only if the required volume is lower than 3 mL and resuspend the cell pellet to the right volume.
- Cell seeding and treatment
- Seed 100 µL of cell suspension per well in a 96-well cell culture plate (1 x 105 cells / well).
NOTE: The cell number for seeding was optimized for PBMC cultures. While low cell concentrations will not yield a sufficient amount of RNA for sequencing, an excessive number of cells will increase the risk of suboptimal cell lysis and inhibition of the reverse transcription reaction. - Prepare drug dilutions at a concentration two times the one used for treatment (2x). Choose the solvent according to the solubility of the compound, preferably complete growth medium.
NOTE: PBS or dimethyl sulfoxide (DMSO) can be used if sufficient solubility cannot be reached otherwise. The maximum concentration of DMSO in the resulting incubation volume should not exceed 0.5 % to prevent cytotoxicity induced by the solvent. - Add 100 µL of the 2x drug dilution (final concentration in well 1X) and incubate at 37 °C for the defined time.
NOTE: Complementary investigations should be carried out in order to establish the optimal drug incubation time and to exclude potential mechanisms of cytotoxicity.
- Seed 100 µL of cell suspension per well in a 96-well cell culture plate (1 x 105 cells / well).
- Cell harvesting and lysis
- Centrifuge the cell culture plate for 10 min (300 x g, 20 °C). Gently remove the supernatant with a vacuum pump keeping the plate at an angle (30°-45°) while aiming the tip at the low corner of the well to remove as little cells as possible.
- Wash the cells by adding 200 µL of ice-cold PBS to each well and centrifuge the plate for 5 min (300 x g, 4 °C). Remove the supernatant with a vacuum pump, again, keeping the plate at a sharp angle to remove as much PBS as possible.
- Prepare the lysis buffer as described in Table 1 for the number of reactions (rxn) needed, adding 10% overage.
- Add 15 µL of lysis buffer to each well and seal the plate with adhesive sealing film to protect the samples against contamination. Vortex the plate before centrifuging for 1 min (1000 x g, 4 °C) and incubate for 5 min on ice.
- Collect 6 µL of lysed cell solution in a PCR plate and shortly freeze the cell lysate at -80 °C to ensure optimal cell lysis. Be sure to avoid multiple freezing cycles of the plates.
NOTE: STOPPING POINT: If cDNA production is not carried out subsequently, the cell lysate can be stored at -80 °C for up to several months without considerable decrease of RNA quality.
3. Library preparation for sequencing
- Reverse transcriptase (RT) reaction
NOTE: When working with RNA, place all samples on ice and be sure to use nuclease-free equipment (sterile, disposable plasticware) and water.- Prepare the RT reaction mix as described in Table 2 for the number of reactions needed, adding 10% overage. Briefly vortex the mix and shortly spin it down. Keep the mix on ice until use.
- Thaw the cell lysate at room temperature (RT) and briefly spin it down to collect all cell lysate at the bottom of the plate.
- Perform mRNA denaturation on a thermocycler as per Table 3.
- Take the plate out of the thermocycler and shortly spin it down to collect potential condensate. Add 6 µL of RT reaction mix into each well for a final volume of 12 µL. Seal the plate to protect the plate and to avoid evaporation, vortex, and spin it down.
- Place the plate on the thermocycler and start the RT reaction program as per Table 3.
- Pre-amplification
- Thaw the high-fidelity DNA polymerase and in-situ PCR (ISPCR) primer at room temperature.
- Prepare the pre-amplification mix as per Table 4 for the number of reactions needed, adding 10% overage. Briefly vortex the mix and spin it down.
NOTE: The enzyme mix is stable at room temperature for hours. - Spin down the PCR plate, add 15 µL of the pre-amplification mix to each well and seal the plate.
- Place it on the thermocycler and start the pre-amplification reaction as per Table 5.
- Adjust the number of cycles due to RNA content. Start with a lower number and increase if cDNA yield is not sufficient.
NOTE: In our experience, optimal number of cycles should be established for each cell type, experimental condition and treatment will not influence the optimal number of cycles. We, therefore, recommend establishing the optimal number of cycles experimentally when establishing this protocol for a new cell type. In general, primary cells will require a higher number of cycles compared to cell lines. STOPPING POINT: The pre-amplification product can be stored at - 20 °C.
- Adjust the number of cycles due to RNA content. Start with a lower number and increase if cDNA yield is not sufficient.
- cDNA clean-up and quality control (QC)
NOTE: Sample clean-up can be performed sequentially for each plate as the samples are rather stable at this stage. Increasing the number of samples will lead to a longer duration of the protocol but will not limit the number of samples that can be processed simultaneously.- Before starting, bring the magnetic purification beads to room temperature and vortex on high speed for 1 min to fully resuspend the beads.
- Add 0.8x v/v (20 µL) magnetic purification beads to each well and incubate at room temperature for 5 min.
- Place the PCR plate on a magnetic rack for 5 min until the beads are fully separated. Remove the supernatant carefully.
- Keeping the plate on the magnetic rack, gently add 100 µL of freshly prepared 80% ethanol to wash the beads and incubate for 30 s. Use a small volume pipette to remove the supernatant. Repeat for an additional washing step. Be sure to remove as much ethanol as possible.
- Air-dry the beads on the magnetic rack at room temperature for up to 5 min until the ethanol is fully evaporated and the beads no longer look shiny.
- Remove the plate from the magnetic rack, resuspend the beads in 20 µL of nuclease-free water and incubate for 2 min.
- Place the plate again on the magnetic rack until the beads are separated.
- Recover the eluate in a new PCR plate for cDNA QC and tagmentation.
- Perform a TapeStation or FragmentAnalyser assay to evaluate the size distribution and concentration of the cDNA library (Recommended: TapeStation D5000 assay). For details, see manufacturer instructions. A typical yield of 20 ng is to be expected.
- Tagmentation with kit
NOTE: Other Tagmentation protocols can be used if already established.- Dilute the cDNA to a final concentration of 150 - 300 pg/µL using nuclease-free water.
- Preprogram the thermocycler as per Table 6 to ensure an immediate start of the tagmentation reaction after adding the cDNA to the enzyme mix.
- Prepare the tagmentation mix as per Table 7 for the number of reactions needed, adding 10% overage.
- Dispense 3 µL of the tagmentation mix per reaction onto a new PCR plate and add 1 µL of cDNA to each reaction.
- Start the tagmentation reaction immediately after adding the cDNA.
- Inactivate the reaction by adding 1 µL of neutralize tagment buffer (NT; alternatively, 0.2% sodium dodecyl sulfate (SDS) can be used).
- Enrichment PCR
- Prepare the enrichment PCR mix Table 7 for the number of reactions, adding 10% overage. (Recommended: Nextera UDI set to prevent index hopping on patterned flow cells (e.g., Illumina NovaSeq 6000)).
- Add 9 µL of the enrichment PCR mix to each reaction for a total volume of 14 µL.
- Run the enrichment PCR program as per Table 8.
NOTE: For the enrichment PCR, 16 cycles are standard. If the cDNA quality is poor, additional cycles can be added.
- Clean-up and QC
- Before starting, bring magnetic purification beads to room temperature and vortex on high speed for 1 min to fully resuspend the beads.
- Add 1.0x v/v (14 µL) magnetic purification beads and incubate at room temperature for 5 min.
- Place the plate on a magnetic rack and wait for 5 min until the beads are fully separated. Remove the supernatant carefully.
- Keeping the plate on the magnetic rack, gently add 100 µL of freshly prepared 80% ethanol to wash the beads and incubate for 30 s. Use a small volume pipette to remove the supernatant. Repeat for an additional washing step. Be sure to remove as much ethanol as possible.
- Air-dry the beads at room temperature for 3 min or until they no longer look shiny.
- Remove the plate from the magnetic rack, resuspend the beads in 20 µL of nuclease-free water and incubate for 2 min.
- Place the plate again on the magnetic rack until the beads separate and recover the eluate in a new PCR plate for library QC.
- Perform a TapeStation or Fragment Analyzer assay to evaluate the size distribution and concentration of the cDNA library (Recommended: TapeStation D1000 high-sensitivity assay). For details, see manufacturer instructions. On average, a yield of 10 ng is to be expected.
NOTE: STOPPING POINT: The PCR product can be stored at - 20 °C.
4. Sequencing and data pre-processing
- Sequencing
NOTE: The following guideline will be applicable to all Illumina instruments for short-read sequencing. If the instrumentation is not available, the sequencing can be performed by an external sequencing facility. Other sequencing approaches could also be used. For simplicity, we chose to report on the most widely used sequencing technology only.
NOTE: The following steps related to software usage describe the procedure on an Illumina NovaSeq6000 sequencer.- Pool uniquely indexed libraries in an equimolar ratio according to the results obtained in step 3.6.8.
- Measure the concentration of the final pool with a high-sensitivity assay to calculate the sample molarity as follows:
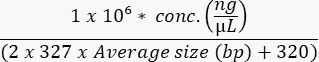
- Load the flow cell according to the instrument's specification and to experimental optimization. Examples for loading concentrations of common instruments are shown in Table 9.
- By touching the screen select Sequence to initiate the run setup.
- Follow the instructions on screen and load flow cell, sequence-by-synthesis cartridge, clustering cartridge, buffer cartridge and ensure the waste containers are empty.
- Once all reagents have been recognized by the instrument click on Run Setup. Define here the run name and the output folder to store the data.
- Define the sequencing details as paired-end with both reads of 51 bp. Two index reads are also sequenced with 8 bp each.
- Press Review and after checking if all sequencing details are correct press Start Run.
NOTE: The recommended sequencing depth is 5 x 106 reads/sample, with a minimum 1 x 106 reads/sample.
- Data pre-processing
- Transform raw sequencing data to FASTQ format and demultiplex according to the sample indexes with the tool Bcl2Fastq2. Perform demultiplexing with default settings. For detailed instructions on Bcl2Fastq2, see the reference manual.
NOTE: FASTQ conversion and demultiplexing are usually performed by the sequencing facility if sequencing is not performed in-house. Sequencing facilities will usually provide demultiplexed FASTQ for further processing. - Several options are available for data alignment and abundance quantification of sequencing reads (Recommended: nf-core RNA-seq pipeline (https://nf-co.re/rnaseq)). The pipeline provides several options, use the default setting with STAR8 as aligned and Salmon9 to quantify the transcript abundance.
- NOTE: For further bioinformatics analysis, several methods are available. It is not in the scope of this protocol to cover all (Recommended: DEseq2 pipeline10). A standard script based on the DEseq2 workflow developed by us can be found on GitHub (https://github.com/jsschrepping/RNA-DESeq2).
- Transform raw sequencing data to FASTQ format and demultiplex according to the sample indexes with the tool Bcl2Fastq2. Perform demultiplexing with default settings. For detailed instructions on Bcl2Fastq2, see the reference manual.
Results
Following the reported protocol, human PBMCs were seeded, treated with different immunomodulatory drugs and, after different incubation times, harvested for bulk transcriptomic analysis using the sequencing protocol (Figure 1).
Ideal drug concentrations and incubation times for test compounds should be identified upstream this protocol with the help of complementary experimental strategies and based on the specific scientific question. In most cases, 2 - 4 h and 2...
Discussion
Drug discovery and drug development can greatly benefit from the holistic view of cellular processes that bulk transcriptomics can provide. Nevertheless, this approach is often limited by the high cost of the experiment with standard bulk RNA-seq protocol, prohibiting its application in academic settings as well as its potential for industrial scalability.
The most critical steps of the protocol are cell thawing and the initial steps of library preparation. Ensuring high viability of the cells...
Disclosures
The authors declare no competing interests.
Acknowledgements
J.L.S. is supported by the German Research Foundation (DFG) under Germany's Excellence Strategy (EXC2151-390873048), as well as under SCHU 950/8-1; GRK 2168, TP11; CRC SFB 1454 Metaflammation, IRTG GRK 2168, WGGC INST 216/981-1, CCU INST 217/988-1, the BMBF-funded excellence project Diet-Body-Brain (DietBB); and the EU project SYSCID under grant number 733100. M.B. is supported by DFG (IRTG2168-272482170, SFB1454-432325352). L.B. is supported by DFG (ImmuDiet BO 6228/2-1 - Project number 513977171) and Germany's Excellence Strategy (EXC2151-390873048). Images created with BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 50 mL conical tube | fisher scientific | 10203001 | |
| Adhesive PCR Plate Seals | Thermo Fisher Scientific | AB0558 | |
| Amplicon Tagment Mix (ATM) | Illumina | FC-131-1096 | Nextera XT DNA Library Prep Kit (96 samples) |
| AMPure XP beads | Beckman Coulter | A 63881 | |
| Betaine | Sigma-Aldrich | 61962 | |
| Cell culture grade 96-well plates | Thermo Fisher Scientific | 260860 | |
| Cell culture vacuum pump (VACUSAFE) | Integra Bioscience | 158300 | |
| Deoxynucleotide triphosphates (dNTPs) mix 10 mM each | Fermentas | R0192 | |
| DMSO | Sigma-Aldrich | 276855 | |
| DTT (100 mM) | Invitrogen | 18064-014 | |
| EDTA | Sigma-Aldrich | 798681 | for adherent cells |
| Ethanol | Sigma-Aldrich | 51976 | |
| Fetal Bovine Serum | Thermo Fisher Scientific | 26140079 | |
| Filter tips (10 µL) | Gilson | F171203 | |
| Filter tips (100 µL) | Gilson | F171403 | |
| Filter tips (20 µL) | Gilson | F171303 | |
| Filter tips (200 µL) | Gilson | F171503 | |
| Guanidine Hydrochloride | Sigma-Aldrich | G3272 | |
| ISPCR primer (10 µM) | Biomers.net GmbH | SP10006 | 5′-AAGCAGTGGTATCAACGCAGAG T-3′ |
| KAPA HiFi HotStart ReadyMix (2X) | KAPA Biosystems | KK2601 | |
| Magnesium chloride (MgCl2) | Sigma-Aldrich | M8266 | |
| Magnetic stand 96 | Ambion | AM10027 | |
| Neutralize Tagment (NT) Buffer | Illumina | FC-131-1096 | Nextera XT DNA Library Prep Kit (96 samples), alternatively 0.2 % SDS |
| Nextera-compatible indexing primer | Illumina | ||
| Nuclease-free water | Invitrogen | 10977049 | |
| PBS | Thermo Fisher Scientific | AM9624 | |
| PCR 96-well plates | Thermo Fisher Scientific | AB0600 | |
| PCR plate sealer | Thermo Fisher Scientific | HSF0031 | |
| Penicillin / Streptomycin | Thermo Fisher Scientific | 15070063 | |
| Qubit 4 fluorometer | Invitrogen | 15723679 | |
| Recombinant RNase inhibitor (40 U/ul) | TAKARA | 2313A | |
| RPMI-1640 cell culture medium | Gibco | 61870036 | If not working with PBMCs, adjust to cell type |
| SMART dT30VN primer | Sigma-Aldrich | 5' Bio-AAGCAGTGGTATCAACGCAGAG TACT30VN-3 | |
| Standard lab equipment | various | various | e.g. centrifuge, ice machine, ice bucket, distilled water, water bath |
| SuperScript II Reverse Transcriptase (SSRT II) | Thermo Fisher Scientific | 18064-014 | |
| SuperScript II Reverse Transcriptase (SSRT II) buffer (5x) | Thermo Fisher Scientific | 18064-014 | |
| Tagment DNA Buffer (TD) | Illumina | FC-131-1096 | Nextera XT DNA Library Prep Kit (96 samples) |
| TapeStation system 4200 | Agilent | G2991BA | |
| Thermocycler (S1000) | Bio-Rad | 1852148 | |
| TSO-LNA (100 uM) | Eurogentec | 5' Biotin AAGCAGTGGTATCAACGCAGAG TACAT(G)(G){G | |
| Vortex-Genie 2 Mixer | Sigma-Aldrich | Z258415 |
References
- Hughes, J. P., Rees, S., Kalindjian, S. B., Philpott, K. L. Principles of early drug discovery. Br J Pharmacol. 162 (6), 1239-1249 (2011).
- Yang, X., et al. High-throughput transcriptome profiling in drug and biomarker discovery. Front Genet. 11, 19 (2020).
- Bonaguro, L., et al. A guide to systems-level immunomics. Nat Immunol. 23 (10), 1412-1423 (2022).
- Carraro, C., et al. Decoding mechanism of action and sensitivity to drug candidates from integrated transcriptome and chromatin state. ELife. 11, 78012 (2022).
- Picelli, S., et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 10 (11), 1096-1098 (2013).
- Picelli, S., et al. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc. 9 (1), 171-181 (2014).
- De Domenico, E., et al. Optimized workflow for single-cell transcriptomics on infectious diseases including COVID-19. STAR Protoc. 1 (3), 100233 (2020).
- Dobin, A., et al. ultrafast universal RNA-seq aligner. Bioinformatics. 29 (1), 15-21 (2013).
- Patro, R., et al. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 14 (4), 417-419 (2017).
- Love, M. I., Huber, W., Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 550 (2014).
- Frankish, A., et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766-D773 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved