A subscription to JoVE is required to view this content. Sign in or start your free trial.
Using Home-based, Remotely Supervised, Transcranial Direct Current Stimulation for Phantom Limb Pain
* These authors contributed equally
In This Article
Summary
The goal of this study is to describe a protocol for the home-based delivery of remotely supervised transcranial direct current stimulation (RS-tDCS) conserving the standard procedures of in-clinic practice, including safety, reproducibility, and tolerability. The participants included will be patients with phantom limb pain (PLP).
Abstract
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that uses low-amplitude direct currents to alter cortical excitability. Previous trials have established the safety and tolerability of tDCS, and its potential to mitigate symptoms. However, the effects are cumulative, making it more difficult to have adherence to the treatment since frequent visits to the clinic or outpatient center are required. Moreover, the time needed for transportation to the center and the related expenses limit the accessibility of the treatment for many participants.
Following guidelines for remotely supervised transcranial direct current stimulation (RS-tDCS) implementation, we propose a protocol designed for remotely supervised and home-based participation that uses specific devices and materials modified for patient use, with real-time monitoring by researchers through an encrypted video conferencing platform. We have developed detailed instructional materials and structured training procedures to allow for self- or proxy-administration while supervised remotely in real time. This protocol has a specific design to have a series of checkpoints during training and execution of the visit. This protocol is currently in use in a large pragmatic study of RS-tDCS for phantom limb pain (PLP). In this article, we will discuss the operational challenges of conducting a home-based RS-tDCS session and show methods to enhance its efficacy with supervised sessions.
Introduction
The sensation of pain and discomfort experienced in an amputated limb and referred to as phantom limb pain (PLP) is a complex condition, challenging to treat, consisting of a refractory nature that contributes to the difficulty in achieving complete and long-lasting pain relief and management. The lack of effective treatment owing to its neuropathic nature, resulting from abnormal nerve activity, or signaling, neural plasticity, psychological factors, and limited understanding and research, influences the complexity of the phenomenon in the pain presentation and the treatment outcomes. From all available treatments, recent studies using transcranial direct current stimulation (tDCS) have reported positive results when combining stimulation of the primary motor cortex (M1) with motor representation techniques1,2,3,4. As Kikkert et al. published in 2019, the long-term effects of the combined stimulation resulted in significant, maintained pain reduction after intervention and a follow-up period of 3 months, with meaningful improvements and large effect sizes in lower-limb amputees.
Although the effects are promising, the clinical translations of these results are limited due to geographical constraints and disabilities related to amputations, which delay and affect the access to adequate post-amputation rehabilitation5. One solution is to deploy these interventions in remote environments using digital technologies and telehealth approaches6. A recent international consensus reported the requirements to successfully implement digitalized electrical stimulation7, including a support team available at all times to manage medical emergencies, cost optimization strategies, implementation of insurance coverage for further field development, specialized teams or third-party services for undertaking the development of software and hardware for the remote use of devices, digital marketing strategies to enhance publicity among potential patients, and front-end interfaces for user experience enhancement.
The adequate implementation of remotely supervised transcranial direct current stimulation (RS-tDCS) protocols has the potential to accelerate the clinical application of this safe and effective intervention4 and facilitate its combination with behavioral modalities that can be performed at home (e.g., physical therapy, mindfulness). Recent studies have shown feasibility and equivalent results with RS-tDCS compared to previous on-site tDCS studies for the same condition8,9. However, practical details and guidance on how to implement RS-tDCS for clinical trials in chronic pain are still limited in the literature. There are open questions on RS-tDCS such as the need for online supervision performed by a trained specialist in the technique compared to the self-administered tDCS therapy after receiving proper coaching. Furthermore, questions remain unanswered regarding metadata registration, adherence to the treatment guidelines, the use of technology such as apps to track contact quality and time of usage, avoiding the misuse of devices related to non-scheduled stimulation sessions, and topics associated with "internet issues"-protection of personal information, recording of health records, rules of sharing, and password protection for access.
Therefore, our goal is to provide a visual guideline on how to perform an RS-tDCS session, as well as a description of the logistics and challenges of its implementation for treating phantom limb pain (PLP) in the context of a pragmatic clinical trial.
Protocol
All procedures were conducted under institutionally approved protocols with patient consent. See Figure 1 for an image of the intervention kit and main components and Figure 2 for the RS-tDCS session structure.
1. Pre-intervention procedures
- Perform recruitment pre-screening according to inclusion and exclusion criteria. Include patients who are adult amputees, who regularly experience phantom pain once a week or more with an intensity level of at least 4 on a Visual analog scale (VAS), and who do not have contra-indications to tDCS or unstable comorbidities.
NOTE: The sample size was calculated according to a calculation on our preliminary results from a previous RCT and our meta-analysis on tDCS effects, considering the minimally clinically significant difference of an effect size of 0.5 (80). We assumed a type I error of 5% and type 2 of 20% using a t-test calculation for differences and expanded to account for a 10% attrition rate. Reaching 145 subjects per group, 290 total. - Enroll the subject in the research study by filling out the registration, including the informed consent form, date of birth, gender, and questions about medical history and amputee profile.
- Perform the screening during the consent visit.
- Set up a date for the baseline and randomization process.
- Baseline visit and training
- Have the patients go through a series of questionnaires regarding pain, sleep, quality of life, cognition, and mental health.
- Randomize the subjects (according to their treatment expectancy) into two groups: either RS-tDCS of the M1 with somatosensory testing (intervention) or usual care (control group).
- Have the patients receive their training according to the randomization, so they can familiarize themselves with the provided materials and practice for the real-time remote supervised procedure.
- Conduct this step in person but do it remotely if the distance and the patient's literacy dictate this. In this case, perform the same described procedures using the same materials but explain how to operate the devices during a Zoom meeting.
- Train the patients from the usual care group in the use of the wearable HRV monitor and its corresponding App installed in a provided tablet, as well as the Zoom platform integrated into a laptop.
- Introduce the subjects to the password-protected laptop, where key features will be explained (including password, switching on/off, and USB webcam connection).
- Show the participants how to join the meeting through a personalized and secured call ID meeting and password via the Zoom platform. Have the participants practice with the researcher at least once.
- To guarantee activity success, ensure that the participants are able to trial a real-in-time session recording of HRV.
- Guide the patients on the correct placement of the HRV wearable device (in the middle of the sternum line).
- Once the sensors have been checked for placement, introduce the participants to the password-protected tablet. Show the participants how to pair the devices, change the record name, start, end the recording, and save it.
NOTE: Ensure that all these previous steps will be performed by the patient with researcher guidance once and then repeated without guidance to guarantee information retention.
- Stimulation group, RS-tDCS
- Show an instructional video and repeat this video content with the patient step-by-step.
- Show the tDCS device to the patient while placed on a mannequin head. Explain the various parts of this device, and give the patient a chance to correctly clip the SNAPpads to the SNAPstrap and into the device.
- Using a mirror, ask the patients to put the SNAPstrap on themselves, as it was on the mannequin's head, until they achieve a comfortable level and make sure the participant is correctly aligning the cathode and the anode as well as the back strap to ensure correct placement.
- Turn on the RS-tDCS device and show the steps to follow until stimulation, how to access the stimulation menu, enter the stimulation code, and what to do in case they need to abort a session.
- Finally, ask the participants to go over all the steps again, either practicing or verbally based on self-assessed competency. Go over the visiting schedule and explain the normal flow of every visit.
- To guarantee training success, fill out a comprehensive checklist covering all essential aspects of training (Table 1).
| 1) Computer |
| · REDCap |
| · Open surveys |
| · Filling and submitting forms |
| · Zoom Log in and out |
| 2) Heart rate monitor |
| · Heart rate monitor app |
| · Correct placement |
| · Recording of the heart rate |
| 3) Home-Based tDCS |
| · Head and electrode preparation |
| · Gather the materials: sponges, head strap, the stimulator device, the saline solution, syringes, and the laptop provided by the Lab. |
| · Find the stimulation area (the central line of your head and your ear) – identified correctly the M1. |
| · Check the skin for redness or any sign of damage. |
| · Open the pre-soaked sponges and attach the electrodes (located inside the pre-soaked sponges) to the head strap. |
| · If the sponges are not wet enough, prepare with the correct amount of saline solution (approx. 6ml on each side). |
| · The sponge in the M1 area. |
| 4) Device preparation and Stimulation |
| · Connect the electrode cable to the tDCS device, according to the matched colors (red cable into the red entrance and the black cable into the black entrance). |
| · Before starting, turn on the computer and open the conference call. |
| · Follow the instructions provided by the research team. |
| · Turn on the device. |
| · Press any button to get the main menu screen. |
| · Press the button to start stimulation. |
| · Check the quality of the setup ( If the quality is not good, tell the team and wait for instructions). |
| · Press the pound button and correctly enter the activation code to start the stimulation. |
Table 1: Training checklist.
2. Study intervention visits
NOTE: The study consists of a total of 23 visits, with 20 sessions involving either stimulation or usual care. Throughout the intervention part, regardless of group allocation, both will be connected to the trained researcher staff via Zoom.
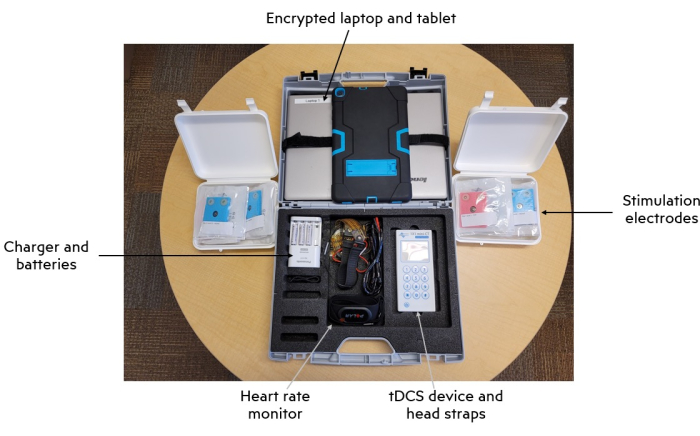
Figure 1: Intervention kit and main components. Abbreviation: tDCS = transcranial direct current stimulation. Please click here to view a larger version of this figure.
- When the patients are at home, connected via Zoom meeting, begin the session by asking the patient about their levels of PLP, phantom limb sensations (PLS), residual limb pain (RLP), and telescoping experienced within the past 24 h by using the VAS score, which is the primary outcome.
- Have the patient begin recording their HRV (measured for the secondary outcome).
- Ensure the correct positioning of the device (middle of the sternum line), as well as its pairing with the tablet to guarantee accurate recordings.
- Remind the patients at the beginning of each session about their research ID and the visit number.
- Have the patients show the tablet screen through the webcam to ensure the recording is being performed accordingly.
- After 5 min, have the patient stop and save the recording by the patient in the App. When questions arise, guide the patients throughout by remaining connected with the patient for the total duration of the session.
- For patients in the intervention group, check their scalp condition prior to stimulation via webcam. If the skin is intact, perform the session. For that, have the patients place the SNAPstrap as shown on the training visit.
- Always position the anode SNAPpad situated on the motor cortex (C3 or C4) contralateral to the amputated limb. Ensure that the cathode SNAPpad is located on the supraorbital space, ipsilateral to the amputation side. Encourage the patients to always use a mirror to facilitate collocation and then, turn on the RS-tDCS device.
- Once they are on the stimulation menu, observe that the screen will show the connection quality rated as poor, moderate, or good. When a connection is poor, the device will beep, indicating that no current can be delivered. In that case, have the patients either rearrange the SNAPpads, check for cable connectivity to the device, or add saline solution. Have the patients press the sponges against their skin for 20 s, thus enhancing contact to solve most cases of poor connectivity and start the stimulation when good connectivity appears.
- Provide a code for single-time use to the patients to avoid non-supervised stimulations. During the training session, follow the study protocol with 2 mA intensity for 20 min with a ramp of 30 s to create the stimulation codes on the patient's device. Ensure that these codes remain blinded to the participant to ensure stimulation is delivered within the study context only.
- Always position the anode SNAPpad situated on the motor cortex (C3 or C4) contralateral to the amputated limb. Ensure that the cathode SNAPpad is located on the supraorbital space, ipsilateral to the amputation side. Encourage the patients to always use a mirror to facilitate collocation and then, turn on the RS-tDCS device.
- Once stimulation has been started, complete a 5 min guided meditation consisting of a body scan technique. Begin somatosensory training by following simple and slow movements of the limbs, including movement of the phantom limb until stimulation is complete.
NOTE: Due to safety reasons, to ensure study protocol adherence, this research protocol warrants constant supervision. When possible, the same researcher will be assigned throughout the study for the same participant. - At the end of each session, administer a questionnaire asking about possible side effects and pain levels on a VAS score to the patient (Supplementary File 1). If a patient's pain level is increased by at least 2 points in the VAS scale, (pre-stimulation vs post-stimulation) in two consecutive visits, stop the intervention completely.
- If at any moment of the stimulation, the patient is experiencing severe discomfort or wants to stop the stimulation, ask the subject to abort the session (previously explained during the training session).
Results
Our home-based and remotely supervised protocol is currently being tested in a large, pragmatic, randomized, clinical trial of patients with PLP. Based on previous clinical trial testing in-clinic tDCS in PLP patients, we expect a reduction in the level of PLP, PLS, and RLP compared to the usual care group. This reduction is expected to reach an effect size of at least 0.5, namely a clinically important difference.
Regarding safety outcomes, our initial exploration has shown a similar safety p...
Discussion
Aspects of training, challenges, and solutions
Given the nature of this research study and the type of intervention, being home-based, some challenges have risen; among them were day-to-day issues such as internet connection, contact quality of the operated device, and getting familiar with the devices. The potential challenges presented by RS-tDCS research have been overcome through several creative solutions. Prior to every session, the internet connection is checked on both ends to minimize inte...
Disclosures
The authors have no conflicts of interest.
Acknowledgements
None
Materials
| Name | Company | Catalog Number | Comments |
| 1 x 1 tDCS mini-CT stimulator | Soterix | parameters preset to two milliamps of stimulation for 20 min | |
| Lenovo Laptop | Lenovo | It contains a headstrap and disposable clip-on sponges for stimulation. A computer with Zoom access, to conduct the RS-tDCS sessions. The Zoom videocalls will be addressed to a secured account by Mass General Brigham (MGB) | |
| Lenovo Smart Tab M8 8'' | Lenovo | We also record the heart rate variability (HRV) and therefore, we provide a tablet with the Polar app installed and the chest HR monitor. | |
| Polar H10 Heart Rate Monitor | POLAR device, in addition to the materials for the RS-tDCS intervention, we also record the heart rate variability (HRV) and therefore we provide a tablet with the Polar app installed and the chest HR monitor. | ||
| Saline solution with a syringe for application over the sponges | |||
| SNAP Headgear accessories | |||
| SNAPstrap, motor left (anode: C3, cathode: supraorbital) or motor right (anode: c4, cathode: supraorbital) according to the side of amputation (contralateral to stimulation) | |||
| SNAPpads, 5 x 7 CMS with pre-inserted carbon rubber snap electrode sites located on the SNAPstrap | |||
| Webcam | to ensure a proper visualization of the electrode placement |
References
- Gunduz, M. E., et al. Effects of combined and alone transcranial motor cortex stimulation and mirror therapy in phantom limb pain: A randomized factorial trial. Neurorehabilitation and Neural Repair. 35 (8), 704-716 (2021).
- Pacheco-Barrios, K., Meng, X., Fregni, F. Neuromodulation techniques in phantom limb pain: A systematic review and meta-analysis. Pain Medicine. 21 (10), 2310-2322 (2020).
- Segal, N., et al. Additive analgesic effect of transcranial direct current stimulation together with mirror therapy for the treatment of phantom pain. Pain Medicine. 22 (2), 255-265 (2021).
- Fregni, F., et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. International Journal of Neuropsychopharmacology. 24 (4), 256-313 (2021).
- Silva-Filho, E., et al. Factors supporting availability of home-based neuromodulation using remote supervision in middle-income countries; Brazil experience. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 15 (2), 385-387 (2022).
- Pacheco-Barrios, K., et al. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Review of Medical Devices. 17 (9), 879-898 (2020).
- Brunoni, A. R., et al. Digitalized transcranial electrical stimulation: A consensus statement. Clinical Neurophysiology. 143, 154-165 (2022).
- Sandran, N., Hillier, S., Hordacre, B. Strategies to implement and monitor in-home transcranial electrical stimulation in neurological and psychiatric patient populations: a systematic review. Journal of Neuroengineering and Rehabilitation. 16 (1), 58 (2019).
- Palm, U., et al. Home use, remotely supervised, and remotely controlled transcranial direct current stimulation: A systematic review of the available evidence. Neuromodulation. 21 (4), 323-333 (2018).
- Van Den Houte, M., Van Oudenhove, L., Bogaerts, K., Van Diest, I., Vanden Bergh, O. Endogenous pain modulation: association with resting heart rate variability and negative affectivity. Pain Medicine. 19 (8), 1587-1596 (2018).
- Cousins, M. J., Lynch, M. E. The Declaration Montreal: access to pain management is a fundamental human right. Pain. 152, 2673-2674 (2011).
- Maceira-Elvira, P., Popa, T., Schmid, A. -. C., Hummel, F. C. Feasibility of home-based, self-applied transcranial direct current stimulation to enhance motor learning in middle-aged and older adults. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 13 (1), 247-249 (2020).
- Tsapkini, K. Home-based transcranial direct current stimulation: Are we there yet. Stroke. 53 (10), 3002-3003 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved