A subscription to JoVE is required to view this content. Sign in or start your free trial.
Automated Compression Testing of the Ocular Lens
In This Article
Summary
We present an automated method for characterizing the effective elastic modulus of an ocular lens using a compression test.
Abstract
The biomechanical properties of the ocular lens are essential to its function as a variable power optical element. These properties change dramatically with age in the human lens, resulting in a loss of near vision called presbyopia. However, the mechanisms of these changes remain unknown. Lens compression offers a relatively simple method for assessing the lens' biomechanical stiffness in a qualitative sense and, when coupled with appropriate analytical techniques, can help quantify biomechanical properties. A variety of lens compression tests have been performed to date, including both manual and automated, but these methods inconsistently apply key aspects of biomechanical testing such as preconditioning, loading rates, and time between measurements. This paper describes a fully automated lens compression test wherein a motorized stage is synchronized with a camera to capture the force, displacement, and shape of the lens throughout a preprogrammed loading protocol. A characteristic elastic modulus may then be calculated from these data. While demonstrated here using porcine lenses, the approach is appropriate for the compression of lenses of any species.
Introduction
The lens is the transparent and flexible organ found in the eye that allows it to focus on different distances by changing its refractive power. This ability is known as accommodation. The refractive power is altered due to the contraction and relaxation of the ciliary muscle. When the ciliary muscle contracts, the lens thickens and moves forward, increasing its refractive power1,2. The increase in refractive power allows the lens to focus on nearby objects. As humans age, the lens becomes stiffer and this ability to accommodate is gradually lost; this condition is known as presbyopia. The mechanism of stiffening remains unknown, at least in part due to the difficulties associated with the biomechanical characterization of the lens.
A variety of methods have been employed to estimate lens stiffness and biomechanical properties. These include lens spinning3,4,5, acoustic methods6,7,8, optical methods such as Brillouin microscopy9, indentation10,11, and compression12,13. Compression is the most accessible experimental technique as it can be performed with simple instrumentation (e.g., glass coverslips14,15) or a single motorized stage. We have previously shown how the biomechanical properties of the lens may be rigorously estimated from a compression test16. This process is technically challenging and requires specialized software not readily accessible to lens researchers interested in relative stiffness measurements. Therefore, in the present study, we focus on accessible methods for estimating the elastic modulus of the lens while accounting for lens size. The elastic modulus is an intrinsic material property related to its deformability: a high elastic modulus corresponds to a stiffer material.
The test itself is a parallel plate compression test and can therefore be performed on suitable commercial mechanical testing systems. Here, a custom instrument was constructed comprised of a motor, linear stage, motion controller, load cell, and amplifier. These were controlled using custom software which also recorded time, position, and load at regular intervals. Pig lenses do not accommodate but are easily accessible and inexpensive17. The following method was developed to incrementally compress the eye lens and quantify its elastic modulus. This method can be easily replicated and will be useful in the study of lens stiffness.
Protocol
Pig eyes were obtained from a local abattoir. No ethical committee approvals were required.
1. Lens dissection (Figure 1)
- Remove all the surrounding tissue from the pig eyes and excess flesh from the sclera, until only the optic nerve remains. Use curved forceps and small dissection scissors to complete this process. Use the nerve as an anchor to hold the eye during dissection.
- Using a scalpel, make a short circumferential cut at the limbus, then another at the equator.
NOTE: This step is performed in this order to avoid damaging the lens and capsule. - Insert microscissors into the cut at the limbus and remove the cornea by lifting the cornea with fine blunt-tipped forceps while cutting around the circumference of the cornea.
- Remove the iris by lifting using blunt-tipped forceps and cut away with micro-scissors.
- Insert dissection scissors into the equatorial cut, then cut circumferentially around the entire equator until the sclera is bisected.
- Once the cut is completed, remove the posterior portion of the sclera. Remove the vitreous gently with forceps, leaving minimal remnants to avoid damaging the lens. If required, cut the vitreous humor coronally to allow the posterior to pull away from the lens and anterior segment.
- Make a meridional cut through the sclera from anterior to posterior using microscissors.
- Beginning at the new meridional cut through the sclera, use microscissors to cut the zonules away from the lens. Using the weight of the lens or edge of the dissection dish, gently stretch the zonules when pulling the lens and sclera apart slightly, allowing the microscissors to cut between the lens and ciliary body, through the zonules, and around the circumference of the lens. This will isolate the lens without damaging the lens capsule if done correctly.
- If desired, remove the capsule using forceps to puncture the capsule at its equator, then peel away the capsule using two forceps.
- Place the lens in phosphate-buffered saline (PBS). Visually inspect the lens for any damage prior to mechanical testing.
2. Lens compression-with/without lens capsule (Figure 2)
NOTE: All steps here with the exception of steps 2.1 and 2.4 are computer-controlled.
- Obtain or construct a parallel plate compression apparatus having a 50 gram-force capacity load cell with the ability to measure displacement on the order of 1 µm.
- Program the motorized stage and load the cell to perform the loading regimen described below (e.g., Supplemental File 1).
- Nearly fill a square box of 1 5/8 inches x 1 5/8 inches with PBS and place it on the compression platform.
- Lower the upper plate into contact with the lower plate to determine the lower limit of motion and absolute gap height.
- Raise the upper plate by ~15 mm.
- Center the lens in the box, taking care that the equatorial plane is horizontal.
- Lower the upper plate close to, but not in contact with, the upper surface of the lens.
- Initiate motion to move the upper plate into contact with the lens, using force feedback with a contact threshold of 3 mN.
- Commence data recording upon determination of contact, recording time, position of the upper plate relative to the lower plate, and force at 500 Hz.
- Apply a preconditioning loading where the lens is compressed by 2.5% of its initial height three times, then 5% three times, then 7.5% three times at a rate of 1%/s.
- Hold the position of the upper plate constant for 1 min after preconditioning.
- Apply a 15% compression at a rate of 1%/s, followed by unloading at the same rate.
- Continue the unloading motion until the upper plate has traveled an additional 2% of the unloaded lens thickness away from the bottom plate to ensure the lens is de-adhered from the upper plate.
3. Estimation of lens modulus
- Estimate the thickness of the lens based on the instrument's gap at the point of contact. Alternatively, use image analysis to measure the thickness from a photograph taken before testing.
- Compute the elastic modulus E using the Hertz model for compression of a sphere between parallel plates (equation [1]; Supplemental File 2).
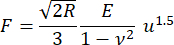 (1)
(1)
Where R is the radius of curvature at the point of contact (assumed equal to half the lens thickness); F is the compression force reported by the load cell;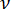 is the Poisson's ratio (assumed equal to 0.5 corresponding to an incompressible material); and u is the downward approach of the upper stage from the point of contact. Note that the elastic modulus and Poisson's ratio are material properties indicating respectively the intrinsic stiffness of the lens and the lens' relative compressibility.
is the Poisson's ratio (assumed equal to 0.5 corresponding to an incompressible material); and u is the downward approach of the upper stage from the point of contact. Note that the elastic modulus and Poisson's ratio are material properties indicating respectively the intrinsic stiffness of the lens and the lens' relative compressibility.
NOTE: This method neglects any role of the lens capsule but does approximately account for the size of the lens, allowing comparison between species.
Results
Six porcine lenses were compressed, first with the capsule intact, then after careful removal of the capsule. Thickness values were 7.65 ± 0.43 mm for encapsulated lenses and 6.69 ± 0.29 mm for decapsulated lenses (mean ± standard deviation). A typical loading history is shown in Figure 3. The resulting force-displacement curves were well-fitted by the Hertz model (i.e., they had a force proportional to the displacement raised to the power of 1.5; Figure 4...
Discussion
Lens compression is a versatile method for estimating lens stiffness. The procedures described above allow comparison between lenses of different species and different sizes. All deformations are normalized against lens size, and the calculation of the elastic modulus approximately accounts for lens size. The effective modulus is considerably higher than the modulus reported previously for the porcine lens4,7,11,
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
Supported by National Institutes of Health grant R01 EY035278 (MR).
Materials
| Name | Company | Catalog Number | Comments |
| Curved Medium Point General Purpose Forceps | Fisherbrand | 16-100-110 | |
| Galil COM Libraries | Galil Motion Control | ||
| High Precision Scalpel Handle | Fisherbrand | 12-000-164 | |
| Linear Stage | McMaster-Carr | 6734K4 0.125" | |
| Load Cell | FUTEK | LSB200-FSH03869 | |
| Load Cell Amplifier | FUTEK | IAA300-FSH03931 | |
| MATLAB | The Mathworks, Inc. | ||
| Microprobe | Surgical Design | 22-079-740 | |
| Miniature Self Opening Precision Scissors | Excelta | 63042-004 | |
| Motion Controller | Galil Motion Control | DMC-31012 | |
| Motor | Galil Motion Control | BLM-N23-50-1000-B | |
| Straight Hemastats | Fine Science | NC9247203 | stainless steel, 14cm |
References
- Gullstrand, A. Helmholtz's treatise on physiological optics. translated edn. The Optical Society of America. , (1924).
- Helmholtz, H. Uber die akkommodation des auges. Arch Ophthalmol. 1, 1-74 (1855).
- Burd, H. J., Wilde, G. S., Judge, S. J. An improved spinning lens test to determine the stiffness of the human lens. Exp Eye Res. 92 (1), 28-39 (2011).
- Reilly, M. A., Martius, P., Kumar, S., Burd, H. J., Stachs, O. The mechanical response of the porcine lens to a spinning test. Z Med Phys. 26 (2), 127-135 (2016).
- Fisher, R. F. The elastic constants of the human lens. J Physiol. 212 (1), 147-180 (1971).
- Erpelding, T. N., Hollman, K. W., O'Donnell, M. Spatially mapping the elastic properties of the lens using bubble-based acoustic radiation force. IEEE Ultrasonics Symp. 1, 613-616 (2005).
- Erpelding, T. N., Hollman, K. W., O'Donnell, M. Mapping age-related elasticity changes in porcine lenses using bubble-based acoustic radiation force. Exp Eye Res. 84 (2), 332-341 (2007).
- Yoon, S., Aglyamov, S., Karpiouk, A., Emelianov, S. A high pulse repetition frequency ultrasound system for the ex vivo measurement of mechanical properties of crystalline lenses with laser-induced microbubbles interrogated by acoustic radiation force. Phys Med Biol. 57 (15), 4871-4884 (2012).
- Scarcelli, G., Kim, P., Yun, S. H. In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys J. 101 (6), 1539-1545 (2011).
- Weeber, H. A., Gabriele, E., Wolfgang, P. Stiffness gradient in the crystalline lens. Graefes Arch Clin Exp Ophthalmol. 245 (9), 1357-1366 (2007).
- Reilly, M. A., Ravi, N. Microindentation of the young porcine ocular lens. J Biomech Eng. 131 (4), 044502 (2009).
- Gu, S., et al. Connexin 50 and AQP0 are essential in maintaining organization and integrity of lens fibers. Invest Ophthalmol Vis Sci. 60 (12), 4021-4032 (2019).
- Sharma, P. K., Busscher, H. J., Terwee, T., Koopmans, S. A., van Kooten, T. G. A comparative study on the viscoelastic properties of human and animal lenses. Exp Eye Res. 93 (5), 681-688 (2011).
- Cheng, C., Gokhin, D. S., Nowak, R. B., Fowler, V. M. Sequential application of glass coverslips to assess the compressive stiffness of the mouse lens: strain and morphometric analyses. J Vis Exp. (111), e53986 (2016).
- Baradia, H., Negin, N., Adrian, G. Mouse lens stiffness measurements. Exp Eye Res. 91 (2), 300-307 (2010).
- Reilly, M. A., Cleaver, A. Inverse elastographic method for analyzing the ocular lens compression test. J Innov Opt Health Sci. 10 (06), 1742009 (2017).
- Hahn, J., et al. Measurement of ex vivo porcine lens shape during simulated accommodation, before and after fs-laser treatment. Invest Ophthalmol Vis Sci. 56 (9), 5332-5343 (2015).
- Parreno, J., Cheng, C., Nowak, R. B., Fowler, V. M. The effects of mechanical strain on mouse eye lens capsule and cellular microstructure. Mol Biol Cell. 29 (16), 1963-1974 (2018).
- Yoon, S., Aglyamov, S., Karpiouk, A., Emelianov, S. The mechanical properties of ex vivo bovine and porcine crystalline lenses: age-related changes and location-dependent variations. Ultrasound Med Biol. 39 (6), 1120-1127 (2013).
- Reilly, M. A., Hamilton, P., Gavin, P., Nathan, R. Comparison of the behavior of natural and refilled porcine lenses in a robotic lens stretcher. Exp Eye Res. 88 (3), 483-494 (2009).
- Mekonnen, T., et al. The lens capsule significantly affects the viscoelastic properties of the lens as quantified by optical coherence elastography. Front Bioeng Biotechnol. 11, 1134086 (2023).
- Wilde, G. S., Burd, H. J., Judge, S. J. Shear modulus data for the human lens determined from a spinning lens test. Exp Eye Res. 97 (1), 36-48 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved