A subscription to JoVE is required to view this content. Sign in or start your free trial.
Three-Dimensional Cell Culture of Adipose-Derived Stem Cells in a Hydrogel with Photobiomodulation Augmentation
In This Article
Summary
Here, we present a protocol demonstrating the use of hydrogel as a three-dimensional (3D) cell culture framework for adipose-derived stem cell (ADSC) culture and introducing photobiomodulation (PBM) to enhance the proliferation of ADSCs within the 3D culture setting.
Abstract
Adipose-derived stem cells (ADSCs), possessing multipotent mesenchymal characteristics akin to stem cells, are frequently employed in regenerative medicine due to their capacity for a diverse range of cell differentiation and their ability to enhance migration, proliferation, and mitigate inflammation. However, ADSCs often face challenges in survival and engraftment within wounds, primarily due to unfavorable inflammatory conditions. To address this issue, hydrogels have been developed to sustain ADSC viability in wounds and expedite the wound healing process. Here, we aimed to assess the synergistic impact of photobiomodulation (PBM) on ADSC proliferation and cytotoxicity within a 3D cell culture framework. Immortalized ADSCs were seeded into 10 µL hydrogels at a density of 2.5 x 103 cells and subjected to irradiation using 525 nm and 825 nm diodes at fluencies of 5 J/cm2 and 10 J/cm2. Morphological changes, cytotoxicity, and proliferation were evaluated at 24 h and 10 days post-PBM exposure. The ADSCs exhibited a rounded morphology and were dispersed throughout the gel as individual cells or spheroid aggregates. Importantly, both PBM and 3D culture framework displayed no cytotoxic effects on the cells, while PBM significantly enhanced the proliferation rates of ADSCs. In conclusion, this study demonstrates the use of hydrogel as a suitable 3D environment for ADSC culture and introduces PBM as a significant augmentation strategy, particularly addressing the slow proliferation rates associated with 3D cell culture.
Introduction
ADSCs are mesenchymal multipotent progenitor cells with the capacity to self-renew and differentiate into several cell lineages. These cells can be harvested from the stromal vascular fraction (SVF) of adipose tissue during a lipoaspiration procedure1. ADSCs have emerged as an ideal stem cell type to use in regenerative medicine because these cells are abundant, minimally invasive to harvest, easily accessible, and well characterized2. Stem cell therapy offers a possible avenue for wound healing by stimulating cell migration, proliferation, neovascularization, and reducing inflammation within wounds3,4. Roughly 80% of the regenerative capacity of ADSCs is attributable to paracrine signaling via their secretome5. Previously, it was suggested a direct local injection of stem cells or growth factors into damaged tissue could illicit sufficient in vivo repair mechanisms6,7,8. However, this approach faced several challenges, such as poor survival and reduced stem cell engraftment within damaged tissues as a result of the inflammatory environment 9. Furthermore, one of the reasons cited was a lack of an extracellular matrix to support the survival and functionality of the transplanted cells10. To overcome these challenges, emphasis is now being placed on the development of biomaterial carriers to support stem cell viability and function.
Three-dimensional (3D) cell culture enhances cell-to-cell and cell-to-matrix interaction in vitro to provide an environment that better resembles the in vivo environment11. Hydrogels have been extensively studied as a class of biomaterial carriers that provide a 3D environment for stem cell culture. These structures are made of water and crosslinked polymers12. Encapsulation of ADSCs in hydrogel has virtually no cytotoxic effect on the cells during culture while maintaining the viability of the cells6. Stem cells cultured in 3D demonstrate enhanced retention of their stemness and improved differentiation capacity13. Similarly, hydrogel-seeded ADSCs demonstrated increased viability and accelerated wound closure in animal models14. Furthermore, hydrogel encapsulation significantly increases the engraftment and retention of ADSCs in wounds15,16. TrueGel3D is made of a polymer, either polyvinyl alcohol or dextran, solidified by a crosslinker, either cyclodextrin or polyethylene glycol17. The gel is a synthetic hydrogel that does not contain any animal products that may interfere with the experiments or trigger an immune reaction during the transplantation of the gel into a patient while effectively mimicking an extracellular matrix18. The gel is fully customizable by altering the composition and individual components. It can house different stem cells and support the differentiation of several cell types by adjusting the stiffness of the gel19. Attachment sites can be created through the addition of peptides20. The gel is degradable by the secretion of metalloproteases, allowing for cell migration21. Lastly, it is clear and allows for imaging techniques.
PBM is a minimally invasive and easily performed form of low-level laser therapy used to stimulate intracellular chromophores. Different wavelengths elicit different effects on cells22. Light in the red to near-infrared range stimulates increased adenosine triphosphate (ATP) and reactive oxygen species (ROS) production by enhancing flux through the electron transport chain23. Light in the blue and green ranges stimulates light-gated ion channels, allowing for the non-specific influx of cations, such as calcium and magnesium, into cells, which is known to enhance differentiation24. The net effect is the generation of secondary messengers that stimulate the transcription of factors triggering downstream cellular processes such as migration, proliferation, and differentiation25. PBM can be used to pre-condition cells to proliferate or differentiate before transplanting the cells into an adverse environment, e.g., damaged tissue26. Pre- and post-transplant PBM (630 nm and 810 nm) exposure of ADSCs significantly enhanced the viability and function of these cells in vivo in a diabetic rat model27. Regenerative medicine requires an adequate number of cells for effective repair of tissues28. In 3D cell culture, ADSCs have been associated with slower proliferation rates compared to two-dimensional cell culture6. However, PBM can be used to augment the 3D cell culture process of ADSCs by enhancing viability, proliferation, migration, and differentiation29,30.
Protocol
NOTE: See the Table of Materials for details related to all materials, reagents, and software used in this protocol. The protocol has been graphically summarized in Figure 1.
1. Two- dimensional (2D) cell culture
NOTE: Immortalised ADSCs (1 x106 cells) are stored at -195.8 °C in liquid nitrogen in a cryopreservation vial containing 1 mL of cell freezing media.
- Preparing the 2D cell recovery medium and 2D culture flask
- Transfer 39 mL of the basal medium into a 50 mL centrifuge tube
- Enrich the basal medium with 20% fetal bovine serum (FBS; 10 mL) and antibiotics (1 mL) (0.5 mL Penicillin-Streptomycin and 0.5 mL Amphotericin B).
- Pre-condition a T75 flask by transferring 6 mL of the cell recovery medium into the flask and incubate it at 37 °C, 5% CO2, and 85% humidity for 45 min (this can be done while performing the cell recovery steps to save time).
NOTE: Warm the complete medium to 37 °C before use and store it at 4 °C for a maximum period of 1 week.
- 2D cell recovery
- Remove a cryovial from the liquid nitrogen storage tank and rapidly defrost the vial in a water bath at 37 °C, until it is completely defrosted.
- Spin down the cryovial using a centrifuge at 1006 x g for 5 min (20 °C) and thereafter remove the supernatant.
- Resuspend the pelleted cells using 1 mL of pre-warmed cell recovery medium.
- Evenly distribute the suspended cells in the pre-conditioned flask (final volume of 7 mL) and incubate at 37 °C, 5% CO2, and 85% humidity.
- After 24 h discard and replace the cell recovery medium.
- Replace the culture medium every 2-3 days until the flask becomes confluent with cells.
- Harvest of 2D culture flask in single cell suspension and cell count
- Remove the 2D culture flask from the incubator and discard the culture media into an appropriate waste container.
- Transfer 10 mL of rinse solution into the flask to rinse the cells and remove waste and protein build-up. After that, discard the rinse solution.
- Add 6 mL of detachment solution to the culture flask to detach the cells. Incubate the flask at 37 °C, 5% CO2, and 85% humidity for 2 min.
NOTE: Observe the flask under a microscope to confirm that all cells have been detached. If not, gently tap on the flask and incubate for another minute. - Once all of the cells have been detached, transfer the cell suspension into a 15 mL centrifuge tube and add 1 mL of the cell recovery media (containing 20% FBS) to the tube. This will neutralize the effect of the detachment solution.
- Centrifuge the tube for 5 min at 1711 x g (20 °C).
- Remove the supernatant and resuspend the cells in 1 mL of complete media.
- Remove 10 µL of the cell suspension, place it in a 500 µL microcentrifuge tube and mix it with 10 µL of trypan blue in a 1:1 ratio.
- Place 10 µL of the trypan blue cell solution mix in a cell counting chamber in duplicate.
- Place the cell counting chamber into the automated cell counter. The cell counter will indicate the total number of cells per mL as well as the number of viable and dead cells. Calculate the average viable cell count and the number of viable cells needed to be embedded in the 3D hydrogel (step 2.2.6).
2. 3D cell culture
- Preparing the 3D complete culture medium
- Transfer 44 mL of the basal medium into a 50 mL centrifuge tube.
- Enrich the media with 10% FBS (5 mL) and antibiotics (1 mL) (0.5 mL Penicillin-Streptomycin and 0.5 mL Amphotericin B).
NOTE: Warm the complete media to 37 °C before use and store it at 4 °C for a maximum of one week.
- Preparing the hydrogel
- Prepare the fast dextran by centrifuging (1000 x g for 30 s at 20 °C) the fast-dextran vial to concentrate the material. Add 175 µL of water to the fast-dextran vial to achieve a final concentration of 30 mmol/L reactive groups.
- Vortex the tube (medium speed for 30 s) containing the fast-dextran solution until the material is fully dissolved. Incubate the dissolved material on ice for 5 min. Centrifuge the tube, briefly vortex it, and keep it on ice during further use.
- Centrifuge the crosslinker (1000 x g for 30 s at 20 °C) to concentrate the material. Add 188 µL of water to the crosslinker vial to achieve a final concentration of 20 mmol/L of thiol groups.
- Vortex the tube (medium speed for 30 s) containing the crosslinker solution until all material is dissolved. Incubate the dissolved crosslinker at room temperature (RT) for 5 min. Centrifuge the tube, briefly vortex, and keep it at RT during further use.
- Adapt the hydrogel protocol from 30 µL to 10 µL for cost reduction (see Table 1 for specific details).
- Prepare the cell suspension to achieve a seeding density of 2.5 x 103 cells per 10 µL hydrogel.
- Combine the components according to Table 1 to create a master mix. Transfer 9 µL of the master mix to a well in a 96-well strip plate.
- Solidify the gel by adding 1 µL of the degradable crosslinker 3 min after the master mix is transferred.
- Cover the hydrogels with 170 µL pre-warmed complete culture media. Incubate for 1 h. After 1 h, replace the culture medium.
NOTE: The hydrogel-embedded cells are ready for further experimentation or analysis within the 3D culture system. Follow additional protocols as needed for downstream applications.
3. Photobiomodulation exposure
- Setting up the laser
- Set up the diode laser system for either green (525 nm) or near-infrared (825 nm) wavelengths. Switch on the lasers and allow them to warm up for the recommended duration.
- Stabilize the power output of the lasers by allowing them to reach a steady state. This ensures consistent and accurate irradiation.
- Measure the power output of the lasers using a laser power meter. Record the power values for each wavelength.
- Use Equation 1 ( Laser irradiation time) to calculate the laser irradiation time. Substitute the values from Table 2 into Equation 1 to determine the appropriate irradiation time for each fluency. In Equation 1, "r" represents the radius of the irradiation zone of the laser.
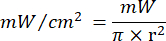
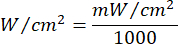
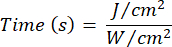
Equation 1 - Refer to Table 2 for the specific parameters needed for the calculation, including fluencies (5 J/cm² or 10 J/cm²) and laser power output.
- Irradiating the 3D cell cultures
- After replacing the complete media (step 2.2.9), place the 96-well plate containing hydrogel-embedded cells under the diode laser system. Irradiate the hydrogels with the calculated laser irradiation time for the selected fluency (5 J/cm² or 10 J/cm²) at the chosen wavelength (green or near-infrared). Therefore, the cultures will only receive a single dose.
- Ensure that each experimental group is accompanied by a control (n = 4), consisting of ADSCs embedded in hydrogel without PBM exposure.
NOTE: Always wear safety glasses appropriate for the wavelength of light you are working with. The lasers are used outside of a safety cabinet; thus, it is crucially important to disinfect the area before use. - Incubate the culture plates at 37 °C, 5% CO2, and 85% humidity for the duration of the experiments. Incubate the plates for 24 h for the first analysis or 10 days for the second analysis period, post-irradiation.
4. Morphology
- Inverted light microscopy
- Turn on the inverted light microscope and allow it to warm up for a few minutes. Ensure the microscope is properly aligned and calibrated.
- Prepare the sample for observation on a microscope slide or a specialized cell culture dish, depending on the experimental setup. Calibrate the microscope for optimal imaging conditions.
- Adjust the focus, brightness, and contrast settings as needed. Select the 20x objective lens on the microscope for observing and recording cell morphology.
- Place the sample on the microscope stage and focus on the cells of interest using the eyepiece. Using the eyepiece or camera view, observe and record the cell morphology. Pay attention to cell shape, size, and any distinctive features.
- Attach the camera module to the microscope. Set up the camera for digital imaging. Ensure proper connection and communication between the camera and the microscope.
- Using the 20x objective lens, capture digital pictures of the observed cells. Use the camera controls to adjust exposure, focus, and other relevant settings.
NOTE: Ensure that the captured images are of high quality and provide a representative view of the cell morphology. - Launch the imaging software on the computer. Capture the image and use the analysis tools within the software to analyze cell morphology.
- Measure cell dimensions, count cells, or perform any relevant analysis based on the experimental objectives.
- Add an appropriate scale bar to the images using the software. Use the known magnification of the microscope objective to accurately represent the scale of the images.
5. Biochemical assays
- Cell recovery
NOTE: The cells need to be recovered from the hydrogel in a single-cell suspension for further downstream biochemical assays.- Prepare the enzymatic cell recovery solution. In a sterile environment, prepare a working solution by diluting the enzymatic cell recovery solution with phosphate-buffered saline (PBS) at a ratio of 1:20 (Solution: PBS). If recovering cells from a specific number of gels or wells is required, calculate the total volume required based on 30 µL of recovery solution per gel.
- Add 30 µL of the working solution to each gel or well containing the cells needing recovery. Ensure thorough and even distribution of the recovery solution by gently rocking or swirling the plate. Incubate the plate with the recovery solution according to the recommended incubation time. This information can typically be found in the product datasheet or protocol provided by the supplier.
- After the incubation period, carefully remove the plate from the incubator. Centrifuge the plates at 1409 x g for 5 min (20 °C) to pellet the cells. Refer to the product datasheet or protocol for recommended centrifugation conditions. Once centrifugation is complete, carefully discard the supernatant, being cautious not to disturb the cell pellet.
- Gently resuspend the pelleted cells in 220 µL of sterile PBS. Ensure thorough mixing to achieve a homogeneous single-cell suspension. The cells are now ready for downstream biochemical assays. Follow the specific protocols for the intended assays, considering the nature of the recovered cells and the requirements of the experimental setup.
- Lactate dehydrogenase (LDH) cytotoxicity assay
- For each well, carefully remove 50 µL of complete culture media, ensuring minimal disturbance to the cell layer.
- Add 50 µL of the cytotoxicity reagent directly to each well containing the remaining 50 µL of culture media. Mix gently by pipetting up and down to ensure thorough mixing of the sample with the reagent.
- Prepare a positive control. Set up triplicate wells with 5 µL of 10x lysis solution per 50 µL of positive control cells included in the kit.
- Incubate the plate at the recommended temperature and duration. This step allows the cytotoxicity reagent to lyse the cells and release LDH into the culture media.
- After the incubation period, add 50 µL of the stop solution directly to each well containing the cytotoxicity reagent and cell lysate. Mix gently by pipetting up and down to ensure proper stopping of the reaction.
NOTE: The stop solution halts further LDH release and ensures the stability of the color development. - Set up the spectrophotometric plate reader according to the manufacturer's instructions. Record the absorbance of each well at 490 nm. This wavelength is specific to the colorimetric detection of the formazan product generated by the LDH reaction.
- Subtract background absorbance readings from both treatment groups and control wells. Obtain background readings from wells containing media and cytotoxicity reagents without cells.
- Analyze the corrected absorbance values for each sample to determine the cytotoxicity level. Higher absorbance values indicate increased LDH release and, consequently, higher cytotoxicity.
- Ensure that each experimental group and control consisted of four repeats (step 3.2.2) for statistical accuracy. Calculate and import the average absorbance of each group and control into an appropriate statistical program.
- Compare the results between treatment groups and appropriate controls to assess the cytotoxic effects accurately and record all absorbance readings, calculations, and experimental conditions for future reference.
- ATP proliferation assay
- In a sterile environment, mix 50 µL of the recovered cells with 50 µL of the ATP reagent in each well.
NOTE: Adjust the volume of cells and reagents based on the number of wells and experimental design. Maintain consistent ratios for accurate results. - Place the plate on a shaker and shake vigorously in the dark for 5 min. This step ensures thorough mixing of the cells with the reagent. After shaking, incubate the plate for an additional 25 min at RT. This incubation period allows the reagent to lyse the cells and generate a luminescent signal proportional to the amount of ATP present.
- Set up the plate reader according to the manufacturer's instructions for luminescence detection. Measure luminescence from each well containing the cell lysate and ATP reagent. Ensure that the plate reader is set to measure luminescence with appropriate settings.
- Subtract background luminescence readings from both treatment groups and control wells. Obtain background readings from wells containing only the ATP reagent and PBS without cells.
- Analyze the corrected luminescence values for each sample to determine ATP levels, reflecting cell proliferation or viability.
- Ensure that each experimental group and control consisted of four repeats (step 3.2.2) for statistical accuracy. Calculate and import the average luminescence of each group and control into an appropriate statistical program.
- Compare the results between treatment groups and relevant controls to assess ATP proliferation accurately. Record all luminescence readings, calculations, and experimental conditions for future reference.
NOTE: For all biochemical analyses in kit form, follow the kit's manual or protocol provided by the manufacturer for specific details, such as recommended incubation times, concentrations, and any additional steps or considerations. Always adhere to laboratory safety guidelines when handling chemicals and biological materials.
- In a sterile environment, mix 50 µL of the recovered cells with 50 µL of the ATP reagent in each well.
Results
To assess the morphology and visually inspect the cell density of the hydrogels, inverse microscopy was used (Figure 2). The ADSCs retained a rounded morphology 24 h after seeding and PBM exposure. The cells were scattered throughout the gel as single cells or in grape-like clusters. The morphology was unchanged after 10 days in 3D culture. No definitive difference in morphology was noted between the experimental groups and controls or between the different experimental groups.
Discussion
ADSCs are an ideal cell type to use for regenerative medicine as they stimulate various processes to aid in wound healing3,4. However, there are several challenges that need to be circumvented, e.g., poor survival rates and ineffective engraftment of the cells in an injury site9. Immortalized cells were used as a commercially available cell line, as they can be passaged for more generations compared to primary cells, they do not need to be...
Disclosures
The authors declare no competing interests.
Acknowledgements
This research was funded by the National Research Foundation of South Africa Thuthuka Instrument, grant number TTK2205035996; the Department of Science and Innovation (DSI) funded African Laser Centre (ALC), grant number HLHA23X task ALC-R007; the University Research Council, grant number 2022URC00513; the Department of Science and Technology's South African Research Chairs Initiative (DST-NRF/SARChI), grant number 98337. The funding bodies played no role in the design of the study, collection, analysis, interpretation of the data or writing the manuscript. The authors thank the University of Johannesburg (UJ) and Laser Research Centre (LRC) for their use of the facilities and resources.
Materials
| Name | Company | Catalog Number | Comments |
| 525 nm diode laser | National Laser Centre of South Africa | EN 60825-1:2007 | |
| 825 nm diode laser | National Laser Centre of South Africa | SN 101080908ADR-1800 | |
| 96 Well Strip Plates | Sigma-Aldrich | BR782301 | |
| Amphotericin B | Sigma-Aldrich | A2942 | Antibiotic (0.5%; 0.5 mL) |
| CellTiter-Glo 3D Cell Viability Assay | Promega | G9681 | ATP reagent, Proliferation assay Kit |
| Corning 2 mL External Threaded Polypropylene Cryogenic Vial | Corning | 430659 | cryovial |
| CryoSOfree | Sigma-Aldrich | C9249 | Cell freezing media |
| CytoTox96 Non-Radioactive Cytotoxicity Assay | Promega | G1780 | Cytotoxicity reagent |
| Dulbecco’s Modified Eagle Media | Sigma-Aldrich | D5796 | Basal medium (39 mL/44 mL) |
| FieldMate Laser Power Meter | Coherent | 1098297 | |
| Flat-bottomed Corning 96 well clear polystyrene plate | Sigma-Aldrich | CLS3370 | |
| Foetal bovine serum | Biochrom | S0615 | Culture medium enrichment (5 mL; 10% / 10 mL; 20%) |
| Hanks Balanced Salt Solution (HBSS) | Sigma-Aldrich | H9394 | Rinse solution |
| Heracell 150i CO2 incubator | Thermo Scientific | 51026280 | |
| Heraeus Labofuge 400 | Thermo Scientific | 75008371 | Plate spinner for 96 well plates |
| Heraeus Megafuge 16R centrifuge | ThermoFisher | 75004270 | |
| Immortalized ADSCs | ATCC | ASC52Telo hTERT, ATCC SCRC-4000 | Passage 37 |
| Invitrogen Countess 3 | Invitrogen | AMQAX2000 | Automated cell counter for Trypan Blue |
| Julabo TW20 waterbath | Sigma-Aldrich | Z615501 | Waterbath used to warm media to 37 °C |
| Olympus CellSens Entry | Olympus | Version 3.2 (23706) | Imaging software: digital image acquisition |
| Olympus CKX41 | Olympus | SN9B02019 | Inverted light microscope |
| Olympus SC30 camera | Olympus | SN57000530 | Camera attached to inverted light microscope |
| Opaque-walled Corning 96 well solid polystyrene microplates | Sigma-Aldrich | CLS3912 | Opaque well used for ATP luminescence |
| Penicillin-Streptomycin | Sigma-Aldrich | P4333 | Antibiotic (0.5%; 0.5 mL) |
| SigmaPlot 12.0 | Systat Software Incorporated | ||
| TrueGel3D – True3 | Sigma-Aldrich | TRUE3-1KT | 10 µL |
| TrueGel3D Enzymatic Cell Recovery Solution | Sigma-Aldrich | TRUEENZ | 01:20 |
| Trypan Blue Stain | Thermo Fisher - Invitrogen | T10282 | 0.4% solution |
| TrypLE Select Enzyme (1x) | Gibco | 12563029 | Cell detachment solution |
| Victor Nivo Plate Reader | Perkin Elmer | HH3522019094 | Spectrophotometric plate reader |
References
- Zuk, P. A., et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 7 (2), 211-228 (2001).
- Yuan, X., et al. Strategies for improving adipose-derived stem cells for tissue regeneration. Burns Trauma. 10, (2022).
- Nilforoushzadeh, M. A., et al. Mesenchymal stem cell spheroids embedded in an injectable thermosensitive hydrogel: An in situ drug formation platform for accelerated wound healing. ACS Biomater Sci Eng. 6 (9), 5096-5109 (2020).
- Yang, M., et al. Thermosensitive injectable chitosan/collagen/β-glycerophosphate composite hydrogels for enhancing wound healing by encapsulating mesenchymal stem cell spheroids. ACS Omega. 5 (33), 21015-21023 (2020).
- Chimenti, I., et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 106 (5), 971-980 (2010).
- Hassan, W., Dong, Y., Wang, W. Encapsulation and 3d culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of peg-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res Ther. 4 (2), 32 (2013).
- Wu, K. H., Mo, X. M., Han, Z. C., Zhou, B. Stem cell engraftment and survival in the ischemic heart. The Ann Thorac Surg. 92 (5), 1917-1925 (2011).
- Lee, K., Silva, E. A., Mooney, D. J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J R Soc Interface. 8 (55), 153-170 (2011).
- Koivunotko, E., et al. Angiogenic potential of human adipose-derived mesenchymal stromal cells in nanofibrillated cellulose hydrogel. Biomedicines. 10 (10), 2584 (2022).
- Dong, Y., et al. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv Funct Mater. 27 (24), 1606619 (2017).
- Tibbitt, M. W., Anseth, K. S. Hydrogels as extracellular matrix mimics for 3d cell culture. Biotechnol Bioeng. 103 (4), 655-663 (2009).
- Mantha, S., et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 12 (20), 3323 (2019).
- Sung, T. -. C., et al. 3D culturing of human adipose-derived stem cells enhances their pluripotency and differentiation abilities. J Mater Sci Technol. 63, 9-17 (2021).
- Garg, R. K., et al. Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. Stem Cells Transl Med. 3 (9), 1079-1089 (2014).
- Kim, Y. M., et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing. Laryngoscope. 124 (3), E64-E72 (2014).
- Dong, Y., et al. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 108, 56-66 (2020).
- Truegel3d hydrogel for 3d cell culture. Merck Available from: https://www.sigmaaldrich.com/ZA/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/3d-cell-culture/truegel3d (2024)
- Braccini, S., Tacchini, C., Chiellini, F., Puppi, D. Polymeric hydrogels for in vitro 3d ovarian cancer modeling. Int J Mol Sci. 23 (6), 3265 (2022).
- Mashinchian, O., et al. In vivo transcriptomic profiling using cell encapsulation identifies effector pathways of systemic aging. eLife. 11, e57393 (2022).
- Matsushige, C., Xu, X., Miyagi, M., Zuo, Y. Y., Yamazaki, Y. Rgd-modified dextran hydrogel promotes follicle growth in three-dimensional ovarian tissue culture in mice. Theriogenology. 183, 120-131 (2022).
- Marx, V. How some labs put more bio into biomaterials. Nat Methods. 16 (5), 365-368 (2019).
- Marques, M. M. Photobiomodulation therapy weaknesses. Laser Dent Sci. 6 (3), 131-132 (2022).
- Hamblin, M. R. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 94 (2), 199-212 (2018).
- Chen, J., et al. Low-level controllable blue LEDs irradiation enhances human dental pulp stem cells osteogenic differentiation via transient receptor potential vanilloid 1. J Photochem Photobiol B. 233, 112472 (2022).
- Chang, S. -. Y., Carpena, N. T., Kang, B. J., Lee, M. Y. Effects of photobiomodulation on stem cells important for regenerative medicine. Med Lasers. 9 (2), 134-141 (2020).
- Bikmulina, P. Y., et al. Beyond 2d: Effects of photobiomodulation in 3d tissue-like systems. J Biomed Opt. 25 (4), 048001 (2020).
- Ahmadi, H., et al. Transplantation of photobiomodulation-preconditioned diabetic stem cells accelerates ischemic wound healing in diabetic rats. Stem Cell Res Ther. 11 (1), 494 (2020).
- Mao, A. S., Mooney, D. J. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A. 112 (47), 14452-14459 (2015).
- De Andrade, A. L. M., et al. Effect of photobiomodulation on the behaviour of mesenchymal stem cells in three-dimensional cultures. Lasers Med Sci. 38 (1), 221 (2023).
- Diniz, I. M., et al. Photobiomodulation of mesenchymal stem cells encapsulated in an injectable rhbmp4-loaded hydrogel directs hard tissue bioengineering. J Cell Physiol. 233 (6), 4907-4918 (2018).
- Carter, M., Shieh, J. C. . Guide to Research Techniques in Neuroscience. , (2015).
- Lutolf, M. P., et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 100 (9), 5413-5418 (2003).
- Robledo, F., et al. Spheroids derived from the stromal vascular fraction of adipose tissue self-organize in complex adipose organoids and secrete leptin. Stem Cell Res Ther. 14 (1), 70 (2023).
- Landry, J., Freyer, J. P., Sutherland, R. M. Shedding of mitotic cells from the surface of multicell spheroids during growth. J Cell Physiol. 106 (1), 23-32 (1981).
- Bogacheva, M. S., et al. Differentiation of human pluripotent stem cells into definitive endoderm cells in various flexible three-dimensional cell culture systems: Possibilities and limitations. Front Cell Dev Biol. 9, 726499 (2021).
- Chen, X., Thibeault, S. L. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-d culture. Acta Biomater. 6 (8), 2940-2948 (2010).
- Crous, A., Van Rensburg, M. J., Abrahamse, H. Single and consecutive application of near-infrared and green irradiation modulates adipose derived stem cell proliferation and affect differentiation factors. Biochimie. 196, 225-233 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved