A subscription to JoVE is required to view this content. Sign in or start your free trial.
Construction of An Orthotopic Xenograft Model of Non-Small Cell Lung Cancer Mimicking Disease Progression and Predicting Drug Activities
In This Article
Summary
This study presents an orthotopic non-small cell lung cancer (NSCLC) model based on intrapulmonary inoculation of multicellular spheroids of fluorescent A549-iRFP cells. The model recapitulates clinical NSCLC stages and responds to cisplatin, according to dynamic in vivo monitoring of long-wavelength fluorescence.
Abstract
Non-small cell lung cancer (NSCLC) is a highly lethal disease with a complex and heterogeneous tumor microenvironment. Currently, common animal models based on subcutaneous inoculation of cancer cell suspensions do not recapitulate the tumor microenvironment in NSCLC. Herein we describe a murine orthotopic lung cancer xenograft model that employs the intrapulmonary inoculation of three-dimensional multicellular spheroids (MCS). Specifically, fluorescent human NSCLC cells (A549-iRFP) were cultured in low-attachment 96-well microplates with collagen for 3 weeks to form MCS, which were then inoculated intercostally into the left lung of athymic nude mice to establish the orthotopic lung cancer model.
Compared with the original A549 cell line, MCS of the A549-iRFP cell line responded similarly to anticancer drugs. The long-wavelength fluorescent signal of the A549-iRFP cells correlated strongly with common markers of cancer cell growth, including spheroid volume, cell viability, and cellular protein level, thus allowing dynamic monitoring of the cancer growth in vivo by fluorescent imaging. After inoculation into mice, the A549-iRFP MCS xenograft reliably progressed through phases closely resembling the clinical stages of NSCLC, including the expansion of the primary tumor, the emergence of neighboring secondary tumors, and the metastases of cancer cells to the contralateral right lung and remote organs. Moreover, the model responded to the benchmark antilung cancer drug, cisplatin with the anticipated toxicity and slower cancer progression. Therefore, this murine orthotopic xenograft model of NSCLC would serve as a platform to recapitulate the disease's progression and facilitate the development of potential anticancer drugs.
Introduction
Among all oncological disorders, lung cancer not only inflicts the highest life loss but also claims the second-highest number of new patients every year in the US1. This devastating malignancy stands as a major obstacle in modern healthcare, urging for a deeper understanding of its intricate biology and more efficacious therapeutic modalities2. Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer and tends to develop into solid tumors3. One of the foremost challenges in lung cancer is the dynamic and heterogeneous tumor microenvironment, which profoundly influences the cancer's progression and responses to therapeutic interventions4,5,6. A deeper understanding of the interplay between cancer cells and their microenvironment at different stages of NSCLC calls for refined pathological models that recapitulate the histological features of NSCLC progression.
In this regard, orthotopic animal models emerge as a promising avenue for NSCLC research. Unlike commonly employed subcutaneous xenograft models7, orthotopic models feature cancer cells that are directly inoculated into the organ of origin. For lung cancer, this means implanting cancer cells directly into the lung tissue8,9. Consequently, orthotopic models of lung cancer better mimic the native tumor microenvironment, including the neighboring tissues, vessels, and immune components, thus improving their physiological and clinical relevance.
Three-dimensional multicellular spheroids (MCS) represent another promising approach to recapitulating features of the tumor environment. Most cancers are characterized by their complex tumor microenvironment, including the various cell-cell interactions, the extracellular matrix, and the gradients in oxygen and nutrients10,11. Traditional 2D cell cultures lack the spatial and structural complexity to recapitulate these tumor-specific features12. In contrast, MCS of appropriate size feature a heterogeneous structure with a hypoxic and necrotic core, which recapitulates not only the intratumoral microenvironment but also the physiological barrier against drug penetration, which is a major mechanism of drug resistance in anticancer therapy13,14,15.
Taking advantage of both the orthotopic animal models and the MCS culturing techniques, MCS have been inoculated to immune-compromised mice to successfully construct orthotopic models of breast cancer and prostate cancer16,17. Herein, we report the detailed methodology to construct and characterize a murine orthotopic xenograft model of lung cancer. This method employs the intrapulmonary inoculation of 3D MCS derived from fluorescent human lung cancer cells (A549-iRFP)18. This model offers an exceptional opportunity to observe the in vivo progression of lung cancer through stages that closely parallel the four clinical stages of NSCLC. Furthermore, the xenograft cancer of this model responded to the clinically established antilung cancer drug, cisplatin.
Protocol
The animal study was performed with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of the Pacific (Animal Protocols 19R10 and 22R10). Eight Male athymic nude mice aged 5-6 weeks, weighing 20-25 g, bred with the referenced Rodent Diet and housed under pathogen-free (SPF) conditions, were used for the present study. Cages, bedding, and drinking water were autoclaved and changed regularly. A schematic of tumor inoculation in mice is shown in Figure 1. See the Table of Materials for details related to all materials and instruments used in this protocol.
1. Establishment of three-dimensional MCS of A549-iRFP cells
- Incubate A549-iRFP cells (human lung adenocarcinoma) in a humidified atmosphere with 5% CO2 at 37 °C and grow them in complete growth medium DMEM containing 10% fetal bovine serum, 1% penicillin-streptomycin, and 1 µg/mL puromycin.
- Seed A549-iRFP cells into 96-well ultra-low attachment, round-bottom spheroid microplate at a seeding density of 4,000 cells/well, using 100 µL of complete growth medium supplemented with 0.3% collagen.
- Centrifuge the microplates at 300 × g for 7 min at 4 °C to facilitate the MCS formation. Examine the MCS morphology under a microscope to ensure cell aggregation after centrifugation.
- After 48 h, add 100 µL of complete growth medium to each well to achieve a total volume of 200 µL of growth medium per well.
- Replace 100 µL of growth medium in each well with fresh complete growth medium every other day.
2. Characterization of A549-iRFP MCS
- Observe and image the MCS morphology using a microscope and estimate the MCS volume by simulation with the referenced software from MATLAB based on the phase-contrast microscope images.
- Open one MCS image in ImageJ software.
- Use Freehand selections to select the edge of the MCS and add it to ROI Manager.
- Click Edit | Selection | Create Mask.
- Click Edit | Invert.
- Click File | Save As | Tiff.
- Open ReViSP software.
- Open the saved TIFF image by clicking Browse.
- Click Start to complete the MCS simulation.
- Measure the iRFP fluorescent signal using the infrared imaging system at the 700 nm channel and quantify the fluorescent signal using software (e.g., Image Studio).
- Open Image Studio software.
- In the Channels tab, select 700 and Intensity = 5.
- In the Scan Controls tab, select 84 µm, Medium, 0.0 mm, and Flip Image. Keep everything else as default.
- Click Start to start scanning.
- Assess and quantify cell viability using the 3D cell viability assay according to the vendor protocol.
- Assess and quantify cellular protein using the BCA Assay according to the vendor protocol.
3. MCS selection for tumor inoculation
NOTE: After MCS are seeded into spheroid microplates and grown for 2-3 weeks with regular growth medium exchange, select MCS with the following appropriate characteristics for tumor inoculation.
- Observe the MCS morphology under a microscope and choose MCS with a round shape with overall smooth edges but with 5-10 rough buddings (arrows in Figure 2F) and a diameter within the range of 700-800 µm.
- Measure the iRFP fluorescence (refer to step 2.2) and select the MCS whose fluorescent signals are within one standard deviation of the average.
4. Intrapulmonary MCS inoculation
NOTE: Use 70% isopropyl alcohol spray to clean the surgical station and the tools before handling the animals. The surgeon should wear sterile gloves throughout the surgical procedures.
- Anesthetize each mouse with an IP injection of 80 mg/kg ketamine and 12 mg/kg xylazine; pinch the mouse feet with forceps to ensure full anesthesia.
- Inject 0.05 mg/kg of buprenorphine hydrochloride subcutaneously to reduce the pain.
- Apply ophthalmic ointment on the eyes to prevent dryness.
- Place the mouse on an infrared heat pad in a dorsal posture and secure the limbs in a stretching position with tapes.
NOTE: Provide heat support to the anesthetized mouse throughout the whole surgical procedure. - Disinfect the back skin by swabbing with alternating rounds of iodine and alcohol, at least three times each.
- Cut a 0.5-1 cm incision using surgical scissors on the left back side (Figure 1B). Carefully use forceps to separate the muscle and fat tissues until the chest wall and lung motion are visible.
- Transfer one selected MCS from a microplate into a glass Petri dish containing ice-cold PBS, using a pipette.
- Attach a 20 G needle onto a 100 µL glass syringe, precool them on ice, and draw 20 µL of a precooled mixture of PBS and Matrigel (1:1 v/v).
- Use the syringe to quickly aspirate one MCS from the Petri dish in a minimum volume of PBS-Matrigel mixture, keeping the MCS in the metal part of the needle.
NOTE: Keep a tight grip on the syringe and plunger to prevent the MCS from slipping out of the needle. - Gently insert the needle vertically between two rib bones to ~3 mm depth and slowly inject all the 20 µL PBS and Matrigel mixture that contains the MCS.
NOTE: Avoid excessive force during the insertion to prevent damage to the lung, vasculature, or heart. - Carefully remove the needle and apply triple antibiotic ointment to the wound.
- Seal the incision with surgical clips, which are to be removed after 2 weeks.
NOTE: Do not suture the incision because the suture lines may interfere with fluorescence imaging. The nude mice are able to heal naturally after surgery. - Place the animal on an infrared heat pad and cover it with laboratory wipes to maintain the body temperature. Monitor the animal for at least 30-60 min until it wakes up and moves properly.
- Inject 0.05 mg/kg of buprenorphine hydrochloride subcutaneously every 8 h for the first 48 h to reduce the pain.
5. Postsurgical monitoring
- Measure the body weight every 3-4 days.
- Measure the iRFP fluorescent signal from the tumor xenograft every 3-4 days on a small animal imaging system at 700 nm channel in four postures: left, right, dorsal, and ventral, while the mice are kept under anesthesia by isoflurane inhalation (flow rate: 1.5-2 L/min oxygen containing 1.5% isoflurane) over 2-3 min.
NOTE: Use 70% isopropyl alcohol spray to clean the small animal imaging system before putting animal onto the imaging station. The surgeon should wear sterile gloves throughout the surgical procedures. - Quantify the net fluorescent intensity of the xenograft cancer in vivo using software (e.g., Image Studio).
- Use a fixed rectangle (A1, 200 x 125 pixels) as the sampling window for the chest area of the mice.
- Use the free-hand function to trace the shape of the mice within the rectangle (A1) and measure the fluorescent intensity (F1) in area A1.
- Use another smaller rectangle (A2, 25 x 40 pixels) to measure the background fluorescence (F2) of the mice in the thigh area.
- Use equation (1) to calculate the net fluorescent intensity of the xenograft cancer in each mouse:
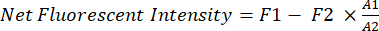 (1)
(1)
Results
Characterization of A549-iRFP MCS
A549-iRFP MCS were successfully cultured in spheroid microplates with the assistance of collagen and centrifugation. When MCS reached a diameter of approximately 500 µm after 1 week, both A549 and A549-iRFP MCS were exposed to a variety of anticancer drugs and formulations for 3 days and then maintained in drug-free growth medium for 4 additional days. The A549-iRFP MCS exhibited a response pattern closely mirroring that of the parent A549 cells. A549 and A549...
Discussion
The construction of A549-iRFP MCS is a straightforward and highly reproducible lab procedure and can be translated to MCS formation for multiple cell lines. The MCS generated with the aid of centrifugation and collagen exhibits a more integral and solid-tumor-like structure within 3-4 days. This method ensures the formation of robust spheroids that maintain their integral structure for extended periods, typically 2-3 weeks or even longer until small buddings begin to emerge. By employing centrifugation and collagen, we s...
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by SAAG and SEED grants from the University of the Pacific. We thank Dr. William Chan for granting access to the Odyssey Infrared Imaging 205 System and Dr. John Livesey for granting access to the SpectraMax iD3 plate reader. We thank Dr. Melanie Felmlee for the technical advice on the animal protocols.
Materials
| Name | Company | Catalog Number | Comments |
| 100 µL Glass Syringe | Hamilton | Part/REF #80601 | |
| 20 G Needle | Thermo Fisher Scientific Inc. | 14 826D | |
| 96-well Ultra-Low-Attachment Spheroid Microplate | Corning | 15-100-173 | |
| A549-iRFP | Imanis Life Sciences | CL082-STAN | |
| AIN-93M Mature Rodent Diet | Research Diets, Inc. | D10012M | |
| Athymic Nude Mouse | Charles River Laboratories, Inc. | Strain Code: 490; homozygous | |
| BCA | Pierce | 23227 | |
| Buprenorphine Hydrochloride | Patterson Veterinary | NDC Number: 42023-179-05 | |
| CellTiter-Glo 3D Cell Viability Assay | Promega | G9683 | |
| Collagen | Gibco | A1064401 | |
| DMEM | Corning | MT10013CV | |
| Fetal Bovine Serum (FBS) | Cytiva HyClone | SH3039603 | |
| ImageJ | Open source tool (https://imagej.net/ij/) | N/A | |
| Image Studio | LI-COR | Version 5.2 | |
| Isoflurane | Patterson Veterinary | NDC Number: 17033-0091-25 | |
| Ketamine | Patterson Veterinary | NDC Number: 50989-0161-06 | |
| Microscope | Keyence | Model number: BZ-X710 | |
| Matrigel | Corning | CB-40234 | |
| Odyssey Infrared Imaging 205 System | LI-COR | Model number: 9140 | |
| PBS | Corning | MT21040CV | |
| Pearl Trilogy small animal imaging system | LI-COR | Model number: 9430 | |
| Penicillin-Streptomycin | Corning | MT30002CI | |
| Puromycin | Thermo Fisher Scientific Inc. | AAJ67236XF | |
| ReViSP software from MATLAB | Open source tool on Sourceforge (https://sourceforge.net/projects/revisp/) | N/A | |
| Surgical Clips--AutoClip System | Fine Science Tools | 12020-00 | |
| Xylazine | Patterson Veterinary | NDC Number: 61133-6017-01 |
References
- Siegel, R. L., Miller, K. D., Jemal, A. Cancer statistics. CA Cancer J Clin. 70 (1), 7-30 (2020).
- Bray, F., et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68 (6), 394-424 (2018).
- Travis, W. D., Brambilla, E., Riely, G. J. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol. 31 (8), 992-1001 (2013).
- Ducote, T. J., et al. Using artificial intelligence to identify tumor microenvironment heterogeneity in non-small cell lung cancers. Lab Invest. 103 (8), 100176 (2023).
- Lim, Z. F., Ma, P. C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 12 (1), 134 (2019).
- Wang, D. C., Wang, W., Zhu, B., Wang, X. Lung cancer heterogeneity and new strategies for drug therapy. Annu Rev Pharmacol Toxicol. 58, 531-546 (2018).
- Peterson, J. K., Houghton, P. J. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 40 (6), 837-844 (2004).
- Madero-Visbal, R. A., et al. Bioluminescence imaging correlates with tumor progression in an orthotopic mouse model of lung cancer. Surg Oncol. 21 (1), 23-29 (2012).
- Mclemore, T. L., et al. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 47 (19), 5132-5140 (1987).
- Zanoni, M., et al. 3d tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci Rep. 6, 19103 (2016).
- Kim, J. B. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 15 (5), 365-377 (2005).
- Chaicharoenaudomrung, N., Kunhorm, P., Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J Stem Cells. 11 (12), 1065-1083 (2019).
- Mehta, G., Hsiao, A. Y., Ingram, M., Luker, G. D., Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 164 (2), 192-204 (2012).
- Huang, B. W., Gao, J. Q. Application of 3d cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J Control Release. 270, 246-259 (2018).
- Hirschhaeuser, F., et al. Multicellular tumor spheroids: An underestimated tool is catching up again. J Biotechnol. 148 (1), 3-15 (2010).
- Sethi, P., et al. 3d tumor tissue analogs and their orthotopic implants for understanding tumor-targeting of microenvironment-responsive nanosized chemotherapy and radiation. Nanomedicine. 11 (8), 2013-2023 (2015).
- Valta, M. P., et al. Spheroid culture of lucap 136 patient-derived xenograft enables versatile preclinical models of prostate cancer. Clin Exp Metastasis. 33 (4), 325-337 (2016).
- Huang, Y., et al. Intrapulmonary inoculation of multicellular spheroids to construct an orthotopic lung cancer xenograft model that mimics four clinical stages of non-small cell lung cancer. J Pharmacol Toxicol Methods. , 106885 (2020).
- Lemjabbar-Alaoui, H., Hassan, O. U., Yang, Y. W., Buchanan, P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 1856 (2), 189-210 (2015).
- Detterbeck, F. C., Boffa, D. J., Kim, A. W., Tanoue, L. T. The eighth edition lung cancer stage classification. Chest. 151 (1), 193-203 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved