A subscription to JoVE is required to view this content. Sign in or start your free trial.
Electroencephalography Measurements in Awake Marmosets Listening to Conspecific Vocalizations
In This Article
Summary
To study the evolution of language, comparing brain mechanisms in humans with those in nonhuman primates is important. We developed a method to noninvasively measure the electroencephalography (EEG) of awake animals. It allows us to directly compare EEG data between humans and animals for the long term without harming them.
Abstract
Vocal communication plays a crucial role in the social interactions of primates, particularly in survival and social organization. Humans have developed a unique and advanced vocal communication strategy in the form of language. To study the evolution of human language, it is necessary to investigate the neural mechanisms underlying vocal processing in humans, as well as to understand how brain mechanisms have evolved by comparing them with those in nonhuman primates. Herein, we developed a method to noninvasively measure the electroencephalography (EEG) of awake nonhuman primates. This recording method allows for long-term studies without harming the animals, and, importantly, allows us to directly compare nonhuman primate EEG data with human data, providing insights into the evolution of human language. In the current study, we used the scalp EEG recording method to investigate brain activity in response to species-specific vocalizations in marmosets. This study provides novel insights by using scalp EEG to capture widespread neural representations in marmosets during vocal perception, filling gaps in existing knowledge.
Introduction
Primates use species-specific vocalizations to convey biologically important information, such as the caller's emotional state or intention to maintain social bonds, the presence of predators, or other dangerous situations. Investigation of the neural mechanisms underlying the perception of vocalization in vocal-rich nonhuman primates may provide us with critical clues to better understand the evolutionary origins of human language.
Common marmosets are small primates native to South America. In recent years, marmosets have been increasingly used as model animals, alongside macaque monkeys, because of their high reproductivity, ease of use owing to their small size, and the development of useful transgenic techniques1,2,3. In addition to their utility as disease models, rich vocal communication within groups is another unique characteristic of this species4,5,6,7. Marmosets routinely exchange vocal signals to communicate with invisible conspecifics in the forest. By examining the brain activity involved in vocal perception and production in marmosets, we can determine how they process the auditory information of their own or conspecific calls in the brain and identify which neural circuits are involved. Previous studies have demonstrated neural activity in the primary auditory cortex8,9,10,11,12 and frontal cortex13,14 involved in vocal production in marmosets. Furthermore, these excited and suppressed neuronal responses were modulated by auditory-vocal interactions in the primary auditory cortex8,10. These studies provided detailed neural activity data at the single-neuron level using invasive recording methods. Numerous studies have further examined the neural activity involved in marmoset vocal production; however, vocal perception remains poorly understood15,16.
Several noninvasive brain imaging studies have elucidated the neural mechanisms of vocal processing in marmosets17,18,19; their high spatial resolution is an advantage, however, keeping animals in the awake state during scanning requires advanced techniques. However, more recently, Jafari et al. identified frontotemporal regions involved in vocal perception in awake marmosets using functional magnetic resonance imaging (fMRI)19. Almost all experiments to elucidate the brain functions involved in vocal perception and production in humans have been conducted using noninvasive methods, such as scalp electroencephalography (EEG), magnetoencephalography (MEG)20,21, and fMRI22,23,24. Numerous studies in humans have investigated brain activity related to vocal perception using EEG. Most of these studies have focused on emotional information25,26,27 and the saliency of emotional words28, with the results revealing changes in event-related potentials during vocal perception29. Electrocorticography (ECoG) and single-neuron recordings using intracranially implanted electrodes in humans have only been conducted in a limited number of experiments in patients undergoing neurosurgical treatment30,31.
An evolutionary perspective comparing humans with monkeys is important when understanding the unique neural mechanisms underlying vocal perception and production that have developed in humans. To directly compare the neural mechanisms involved in speech perception and vocalization in vocal-rich nonhuman primates, such as the marmoset, with humans, it is important to compare data between the two species using the same method. Functional MRI allows whole-brain imaging and has a high spatial resolution. It has the advantage of recording activity perpendicular to the skull or in deep regions that are difficult to record with EEG or MEG. However, the MRI machine is expensive to install and maintain, and there are many restrictions on the stimuli that can be presented due to the nature of the device. In comparison, EEG, event-related potentials (ERPs), and MEG have a high temporal resolution, making them useful for analyzing time-series vocal processing. In particular, EEG has the advantages of high mobility and the ability to be used in a variety of experimental settings, relatively low cost, and the requirement for just a single operator.
Since a large amount of EEG data has already been obtained in humans, EEG measurement methods using non-invasive paradigms are needed for non-human primates. Our research group developed a unique noninvasive EEG recording method using tubes32 for macaques and marmosets. Here, we report several novel findings regarding auditory processing in nonhuman primates33,34,35,36,37. To characterize brain activity in response to species-specific vocalizations in marmosets, we constructed an experimental system to noninvasively record brain activity using electrodes placed on the scalp. In this study, we describe the EEG measurement method for marmosets.
Protocol
All experiments were approved by the Animal Experimentation Committee of EHUB (No.2022-003, 2023-104) and conducted in accordance with the Guide for Care and Use of Laboratory Primates published by EHUB. Nine common marmosets (Callithrix jacchus, six males and three females, 2-12 years old, weighing 330-490 g) were used for the experiment. Four of the nine marmosets were housed in pairs or families, while five were housed individually. Marmosets in the colony typically remain in family housing until two years of age, after which they are transitioned to either pair or individual housing based on the success of pair formation.
1. Animals
- House the marmosets in single cages equipped with nest boxes, wooden perches, and other enrichment devices.
- Maintain the rooms under a 12 h light-dark cycle, with temperature and humidity maintained at 28 ± 2 °C and 40 ± 20%, respectively.
- Feed the animals 14 g of New World monkey pellets twice a day, supplemented with food such as gum arabic and mealworms. Provide water ad libitum.
- Perform all the experiments in a sound-attenuated box in an experimental room.
2. Equipment (Figure 1B and Table of Materials)
- Use 4 mm silver electrodes. The AgCl coating on the electrode surface prevents polarization and ensures stable recording.
- Use an amplifier to record the EEG signals. Bandpass filter (0.016-250 Hz) and sample the data at 1,000 Hz.
- Connect a 64-channel Electrode Input Box to the amplifier, placing it in front of the subject.
- Place the speaker 30 cm from the marmoset head and control the sound level at 65-75 dB, as measured in the ear position. Deliver the auditory stimuli via this speaker.
- Place the camera in front of the subject to monitor their condition during the EEG recording.
- The primate chair is constructed of acrylic plates and synthetic resin posts. For the experiments, have the researcher hold the animals as they sit on the footplate; at this point, insert a neckpiece and secure it to the neck panel, and insert and secure the waist piece to the waist panel. Ensure that the entire body of each marmoset is loosely secured.
3. Anesthesia
- Anesthetize the animals with an intramuscular injection of alfaxalone (6-8 mg/kg) and atropine (0.05 mg/kg). This protocol allows anesthesia to be maintained for approximately 20 min. Administer additional doses of alfaxalone to prolong the duration of anesthesia if the procedure is longer. Also administer antiemetic agents (Maropitant 1 mg/kg, subcutaneous injection) beforehand to counter the nausea, which is a side effect of alfaxalone.
NOTE: Marmosets must be under injection or inhalation anesthesia during the procedures and should be allowed to recover immediately after hair shaving. - Monitor the vitals with a pulse oximeter and administer oxygen-air mixture (O2 0.5 L/min, air 0.5 L/min) if necessary.
- Maintain the room temperature above 27 °C, and wrap a warming cloth around the body of the marmoset to prevent hypothermia.
4. Hair removal
- Shave the entire head (including behind the auricularia) with an electric shaver.
- Apply hair removal cream for sensitive skin. Wipe off the cream with a wet gauze after 5 min.
5. Mask preparation
- Process a thermoplastic mask in advance to fit the size of the monkey chair. Specifically, drill the part of the back that protrudes from the vertical length of the chair with four screw holes such that it can be fixed to the chair's neck plate.
NOTE: Thermoplastic masks are safe and can be used to immobilize the head during radiation therapy in patients. We used a small mask designed for children and cut it to fit the size of the marmosets. This mask softens when placed in warm water at 75 °C and hardens as the temperature reduces when it is removed from the water. - For the experiments, place the animals in a primate chair. Support the anesthetized animal using neck and waist plates and secure the animal and these plates to the chair.
- Warm the mask in hot water and then mold it to fit the head of the marmoset. After removing the mask from the warm water, wait for the temperature to drop (approximately in the 50s °C range) to prevent burns, and then place it on the subject's head to mold the mask.
- After cooling and hardening, detach the mask from the animal and cut out the top of the head and ear parts of the mask to expose the area for the electrode setting.
NOTE: Once this mask was made, it could be used on other individuals.
6. Chair and mask adaptation (30 min/day for 3 days)
- Habituate the animal to the chair under awake conditions by placing it in a chair and rewarding it for approximately 30 min. Repeat this procedure for 3 days.
- Habituate the animal to head fixation using the mask for 2 days.
NOTE: This adaptation process was customized to the individual's condition. After the adaptation, the animals may resist temporarily during capture and head-mask setting, but once they are seated in the chair and their head is fixed, they become calm. - During chair adaptation, evaluate the following behavioral parameters: i) emission of anxious or alarming noises, ii) rejection of the offered reward, and iii) violent movements. If any of these behaviors are observed, terminate the session for that day, allowing the marmoset to gradually adapt to the experimental environment.
7. EEG recording (2 h/day)
- Subject preparation
- Attach a transfer cage to a small window of the home cage and move the marmoset from the home cage to the transfer cage, usually by itself. Cover the carrying cage with a cloth and transfer it to the experimental room.
- Head fixation
- Capture the marmoset using protective gloves and place it in the special chair.
- Place the prepared mask on the marmoset's head. Pass the screw body attached to the chair through the hole in the mask and fix it using thumb screws.
- Secure the head and mask lightly with a band just below the nasion.
NOTE: The subject's head did not need to be completely fixed as long as the electrodes were not removed by movement. Therefore, we used the same mask across all subjects.
- Definition of the location of electrodes
- Use the nasion, inion, and earlobes as anatomical landmarks to determine the location of the electrodes according to the International 10-20 method32,33,34,35,36,37. Measure the distance between the nasion and inion in the mid-line with a tape measure. Define the location of Cz in the middle of the distance. Position the other electrodes (Fz, Pz, Oz, F3, or F4) with 20% of the inion-neion length as the electrode spacing. Mark the electrode positions on the scalp using an oil-based dermatograph.
- Skin preparation
- Rub the marked area with a thin cotton swab dipped in rubbing alcohol to remove dirt and sebum from the scalp.
- Electrode settings
NOTE: Perform steps 7.2.1-7.2.3 at all electrode positions to place electrodes at Fz, Cz, C3, C4, Pz, A1, A2, and F3 (or F4).- Using a tube cutter, cut a piece of silicon tubing with an inner diameter of 4 mm and an outer diameter of 7 mm into a length of approximately 20 mm.
- Apply adhesive to the edges of the cut tube and adhere it to the scalp.
- Fill the inside of the tube with EEG gel using a syringe and a non-pointed syringe needle.
- Connect the reference electrode to Pz and the ground electrodes to F3 or F4 (Figure 1C).
- Insert electrodes into the tube and connect the electrode cable to the input box.
- Launch the application for EEG recordings to measure electrode impedance, and adjust the parameters to ensure all electrodes are below 5 kΩ.
- Bundle the electrode cables to reduce noise.
- Give rewards during the head immobilization procedure and between task sessions by manually administering 1-3 mL of the liquid reward (gum or nutrition) using a syringe.
- Specify the file to be saved on the EEG recording software and press the Start Recording button. Run the script for stimulus presentation immediately after the EEG recording is started. Press the stop button on the EEG recording when the stimulus presentation script execution is complete to end all recordings. When the recording is completed, remove the electrodes and head mask.
- Capture the animals and return them to their carrying cages. Leave the tubes on the scalp; all the tubes fell off naturally in approximately 1 day.
NOTE: In the early periods, we used acetone to dissolve the adhesive glue and remove the tube after the recording sessions; however, there were cases of skin injury; therefore, we subsequently returned the animals to their home cages without removing the tube. In the dozens of experiments, we have conducted thus far, marmosets have not shown any interest in the removed tubes, and no accidental ingestion has ever occurred. - Stimulus
- Record natural simple and compound calls in the captive or experimental room from marmosets that are not used in subsequent EEG recordings. Extract three species-specific simple and compound calls (Phee, Tsik-Ek, and Tsik-String5 calls) from the recorded files.
- In addition to the call stimuli, create white noise using a function in the programming software and use that as a stimulus.
- To follow this protocol, use three auditory files (16 bit, 48 kHz) of marmoset calls in the experiment. Control the task using a custom script.
NOTE: Each block contains 50 calls for each stimulus, for a total of 200 calls. Each recording block will last approximately 10 min. Each participant must perform two blocks. The inter-call interval is 3 s. Of the four audio stimulus files (see Supplemental File 1), the Phee call stimulus was approximately 2 s long, whereas the other three were approximately 1 s long. This was because a single Phee call is still a long-lasting call (Figure 1D). - In each sound stimulus file, the right channel contains the marmoset call or noise data, and the left channel contains the trigger signal for the onset of the stimulus. Send this trigger signal to the EEG recording system via a synchronization device and record it as event time.
8. Data analysis
NOTE: The original code written in the Programming software and toolbox was used to postprocess the EEG data, as outlined below (Supplemental File 2)37.
- Preprocessing
- Re-reference to the linked ear reference.
- High-pass filter at 2 Hz.
- Epoch from 100 ms before to 1000 ms after stimulus onset.
- Baseline-correct to the mean of the 100-ms prestimulus period. Reject artifacts using a criterion of ±150 µV.
- Plotting event-related potential (ERP)
- Average all trial data for each subject.
- Obtain group-averaged waveforms by averaging all subjects.
- To compare the averaged ERPs between call types and noise stimuli, apply a one-way analysis of variance (ANOVA) with stimuli as the between-subjects factor in Cz response.
- Apply post hoc multiple comparison analysis with Tukey's method.
- Plotting event-related spectral perturbation (ERSP)
- Calculate the ERSP to visualize the mean event-related change in spectral power over time at a broad frequency range using Equation (1). Fk (f,t) is the spectral estimate of trial k at frequency f and time t:
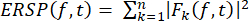 (1)
(1) - Apply time-frequency decomposition to the activities using sinusoidal wavelet transforms, with three cycles of length at the lowest frequency (10 Hz), increasing linearly with frequency up to 32 cycles at the highest frequency (120 Hz).
- Define the initial and transient responses in a 150 ms period after stimulus onset at 2-30 Hz, and the sustained responses in an 800 ms period from 151 to 950 ms after stimulus onset at 40-100 H.
- To test the differences in initial and sustained responses in the Fz and Cz among subject ages and call types, perform a two-way ANOVA using call type as the within-subject factor and ages as the between-subject factor.
- Calculate the ERSP to visualize the mean event-related change in spectral power over time at a broad frequency range using Equation (1). Fk (f,t) is the spectral estimate of trial k at frequency f and time t:
Results
First, we plotted the average event-related potentials (ERPs) for each auditory stimulus in the marmosets (Figure 2). The auditory evoked potential (AEP) was prominent in the Noise condition, reflecting the clear onset of the stimuli (see Figure 1D). To compare the averaged ERPs between call types and noise stimuli, we applied a one-way analysis of variance (ANOVA) with stimuli as the between-subjects factor in Cz response. We found a significant main e...
Discussion
Points to note about anesthesia
Both ketamine and xylazine administration have been attempted, and while these are analgesic and therefore suitable for long painful tasks, marmosets tend to experience decreases in blood oxygen levels without oxygen inhalation44. In short, alfaxalon is probably best suited for painless tasks such as shaving or mask making. In addition, for shaving-, which takes only 10-15 min, inhalation anesthesia would be the most suitable. Isoflurane was n...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by the Hakubi Project of Kyoto University, Grant-in-Aid for Challenging Research (Pioneering) (No.22K18644), Grant-in-Aid for Scientific Research (C) (No. 22K12745 ), Grant-in-Aid for Scientific Research (B) (No. 21H02851), and Grant-in-Aid for Scientific Research (A) (No. 19H01039). We would like to thank Editage (www.editage.jp) for English language editing.
Materials
| Name | Company | Catalog Number | Comments |
| Alfaxalone | Meiji Animal Health | Alfaxan | |
| Amplifier | Brain Products | BrainAmp | |
| Atropine | Fuso Pharmaceutical Industries | Atropine Sulfate Injection | |
| Audio editor | Adobe | Adobe Audition | |
| Data processing software | MathWorks | MATLAB | version R2023a |
| Data processing toolbox | University of California-SanDiego | EEGLAB | |
| Data processing toolbox | University of California-Davis | ERPLAB | |
| Electric shaver | Panasonic | ER803PPA | |
| Electrode | Unique Medical | UL-3010 | AgCl coated (custom) |
| Electrode gel | Neurospec AG | V16 SuperVisc | |
| Electrode input box | Brain Products | EIB64-DUO | 64ch |
| Glue | 3M | Scotch 7005S | |
| Hair removering cream | Kracie | epilat for sensitive skin | |
| Isoflurane | Bussan Animal Health | ds isoflurane | |
| Liquid gum | San-ei Yakuhin Boeki | Arabic Call SS | Gum arabic+water |
| Liquid nutrition | Nestlé Health Science Company | Isocal 1.0 Junior | Polymeric formula |
| Maropitant | Zoetis | Cerenia injectable solution | |
| Monitor Camera | Intel | RealSense LiDAR Camera L515 | |
| Monkey pellets | Oriental Yeast | SPS | |
| Primate chair | Natsume Seisakusho | Order made | |
| Pulse oximeters | Covident | Nellcor | PM10N |
| Skin prepping pasta | Mammendorfer Institut für Physik und Medizin | NeuPrep | |
| Slicon tube | AsONE | Φ4 x 7mm | |
| Speaker | Fostex | PM0.3 | |
| Synchronization device | Brain Vision | StimTrak | |
| Thermoplastic mask | CIVCO | MTAPU Type Uniframe Thermoplastic Mask 2.4mm |
References
- Miller, C. T., et al. Marmosets: A neuroscientific model of human social behavior. Neuron. 90 (2), 219-233 (2016).
- Okano, H., Hikishima, K., Iriki, A., Sasaki, E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 17 (6), 336-340 (2012).
- t'Hart, B. A., Abbott, D. H., Nakamura, K., Fuchs, E. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discov Today. 17 (21-22), 1160-1165 (2012).
- Bezerra, B. M., Souto, A. Structure and usage of the vocal repertoire of Callithrix jacchus. Int J Primatol. 29 (3), 671-701 (2008).
- Agamaite, J. A., Chang, C. J., Osmanski, M. S., Wang, X. A quantitative acoustic analysis of the vocal repertoire of the common marmoset (Callithrix jacchus). J Acoust Soc Am. 138 (5), 2906-2928 (2015).
- Pistorio, A. L., Vintch, B., Wang, X. Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus). J Acoust Soc Am. 120 (3), 1655-1670 (2006).
- Grijseels, D. M., Prendergast, B. J., Gorman, J. C., Miller, C. T. The neurobiology of vocal communication in marmosets. Ann N. Y. Acad Sci. 1528 (1), 13-28 (2023).
- Eliades, S. J., Wang, X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 453 (7198), 1102-1106 (2008).
- Eliades, S. J., Wang, X. Comparison of auditory-vocal interactions across multiple types of vocalizations in marmoset auditory cortex. J Neurophysiol. 109 (6), 1638-1657 (2013).
- Eliades, S. J., Wang, X. Contributions of sensory tuning to auditory-vocal interactions in marmoset auditory cortex. Hear Res. 348, 98-111 (2017).
- Eliades, S. J., Wang, X. Neural correlates of the lombard effect in primate auditory cortex. J Neurosci. 32 (31), 10737-10748 (2012).
- Tsunada, J., Wang, X., Eliades, S. J. Multiple processes of vocal sensory-motor interaction in primate auditory cortex. Nat Commun. 15 (1), 3093 (2024).
- Miller, C. T., Thomas, A. W., Nummela, S. U., de la Mothe, L. A. Responses of primate frontal cortex neurons during natural vocal communication. J Neurophysiol. 114 (2), 1158-1171 (2015).
- Roy, S., Zhao, L., Wang, X. Distinct neural activities in premotor cortex during natural vocal behaviors in a New World Primate, the common marmoset (Callithrix jacchus). J Neurosci. 36 (48), 12168-12179 (2016).
- Wang, X., Merzenich, M. M., Beitel, R., Schreiner, C. E. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 74 (6), 2685-2706 (1995).
- Zeng, H. -. h., et al. Distinct neuron populations for simple and compound calls in the primary auditory cortex of awake marmosets. National Science Review. 8 (11), nwab126 (2021).
- Sadagopan, S., Temiz-Karayol, N. Z., Voss, H. U. High-field functional magnetic resonance imaging of vocalization processing in marmosets. Sci Rep. 5, 10950 (2015).
- Kato, M., et al. Individual identity and affective valence in marmoset calls: in vivo brain imaging with vocal sound playback. Anim Cogn. 21 (3), 331-343 (2018).
- Jafari, A., et al. A vocalization-processing network in marmosets. Cell Rep. 42 (5), 112526 (2023).
- Papanicolaou, A. C., et al. Differential brain activation patterns during perception of voice and tone onset time series: a MEG study. Neuroimage. 18 (2), 448-459 (2003).
- Capilla, A., Belin, P., Gross, J. The early spatio-temporal correlates and task independence of cerebral voice processing studied with MEG. Cereb Cortex. 23 (6), 1388-1395 (2012).
- Belin, P., Zatorre, R. J., Lafaille, P., Ahad, P., Pike, B. Voice-selective areas in human auditory cortex. Nature. 403 (6767), 309-312 (2000).
- Perrodin, C., Kayser, C., Abel, T. J., Logothetis, N. K., Petkov, C. I. Who is that? Brain networks and mechanisms for identifying individuals. Trends Cogn Sci. 19 (12), 783-796 (2015).
- Pernet, C. R., et al. The human voice areas: Spatial organization and inter-individual variability in temporal and extra-temporal cortices. Neuroimage. 119, 164-174 (2015).
- Chen, X., Pan, Z., Wang, P., Zhang, L., Yuan, J. EEG oscillations reflect task effects for the change detection in vocal emotion. Cogn Neurodyn. 9 (3), 351-358 (2015).
- Hiyoshi-Taniguchi, K., et al. EEG correlates of voice and face emotional judgments in the human brain. Cogn Comput. 7 (1), 11-19 (2015).
- Lévêque, Y., Schön, D. Listening to the human voice alters sensorimotor brain rhythms. PloS One. 8, e80659 (2013).
- Liu, T., et al. Electrophysiological insights into processing nonverbal emotional vocalizations. NeuroReport. 23, 108-112 (2012).
- Bruneau, N., et al. Early neurophysiological correlates of vocal versus non-vocal sound processing in adults. Brain Res. 1528, 20-27 (2013).
- Flinker, A., et al. Single-Trial speech suppression of auditory cortex activity in humans. J Neurosci. 30, 16643-16650 (2010).
- Greenlee, J. D. W., et al. Human auditory cortical activation during self-vocalization. PloS One. 6 (3), e14744 (2011).
- Itoh, K., Konoike, N., Iwaoki, H., Igarashi, H., Nakamura, K. A novel "dip-in electrode" method for electrode application to record noninvasive scalp electroencephalograms and evoked potentials in an awake common marmoset. Neuroimage: Reports. 2 (3), 100116 (2022).
- Itoh, K., et al. Cerebral cortical processing time is elongated in human brain evolution. Sci Rep. 12 (1), 1103 (2022).
- Itoh, K., Nejime, M., Konoike, N., Nakamura, K., Nakada, T. Evolutionary elongation of the time window of integration in auditory cortex: macaque vs. human comparison of the effects of sound duration on auditory evoked potentials. Front Neurosci. 13, 630 (2019).
- Itoh, K., Iwaoki, H., Konoike, N., Igarashi, H., Nakamura, K. Noninvasive scalp recording of the middle latency responses and cortical auditory evoked potentials in the alert common marmoset. Hear Res. 405, 108229 (2021).
- Itoh, K., Nejime, M., Konoike, N., Nakada, T., Nakamura, K. Noninvasive scalp recording of cortical auditory evoked potentials in the alert macaque monkey. Hear Res. 327, 117-125 (2015).
- Konoike, N., et al. Comparison of noninvasive, scalp-recorded auditory steady-state responses in humans, rhesus monkeys, and common marmosets. Sci Rep. 12 (1), 9210 (2022).
- Eliades, S. J., Miller, C. T. Marmoset vocal communication: Behavior and neurobiology. Dev Neurobiol. 77 (3), 286-299 (2017).
- Ray, S., Hsiao, S. S., Crone, N. E., Franaszczuk, P. J., Niebur, E. Effect of stimulus intensity on the spike-local field potential relationship in the secondary somatosensory cortex. J Neurosci. 28 (29), 7334-7343 (2008).
- Leonard, M. K., et al. Large-scale single-neuron speech sound encoding across the depth of human cortex. Nature. 626 (7999), 593-602 (2024).
- Dubey, A., Ray, S. Comparison of tuning properties of gamma and high-gamma power in local field potential (LFP) versus electrocorticogram (ECoG) in visual cortex. Sci Rep. 10 (1), 5422 (2020).
- Ray, S., Crone, N. E., Niebur, E., Franaszczuk, P. J., Hsiao, S. S. Neural correlates of high-gamma oscillations (60-200 Hz) in Macaque local field potentials and their potential implications in electrocorticography. Journal Neurosci. 28 (45), 11526-11536 (2008).
- Buzsáki, G., Anastassiou, C. A., Koch, C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13 (6), 407-420 (2012).
- Konoike, N., Miwa, M., Ishigami, A., Nakamura, K. Hypoxemia after single-shot anesthesia in common marmosets. J Med Primatol. 46 (3), 70-74 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved