A subscription to JoVE is required to view this content. Sign in or start your free trial.
Setting a Successful Sorting for Extracellular Vesicle Isolation
In This Article
Summary
This protocol provides a detailed description of sorting extracellular vesicles (EVs) released by mesenchymal stromal cells. In particular, it focuses on the instrument setting and the optimization of the sorting conditions. The goal is to sort extracellular vesicles while preserving their characteristics.
Abstract
Extracellular vesicles (EVs) released by mesenchymal stromal cells (MSCs) contain a set of microRNAs with regenerative and anti-inflammatory roles. Therefore, purified MSC-EVs are envisioned as a next-generation therapeutic option for a wide array of diseases. In this protocol, we report the strategy for successfully sorting EVs from the supernatant of adipose-derived MSCs (ASCs), often used in orthopedics regenerative medicine applications.
First, we described the sample preparation, focusing on EV isolation and labeling steps with carboxyfluorescein succinimidyl ester (CFSE) for fluorescence detection; subsequently, we detailed the sorting process, which constitutes the main part of the protocol.
In addition to the rules defined by MISEV 2023 and MIFlowCyt EV guidelines, we applied specific experimental conditions concerning nozzle size, frequency, and sheath pressure. Morphological parameters are established using beads of diameters selected to cover the theoretical range of EV size. After ASC-EVs sorting, we performed a purity check of the sorted fraction by re-analyzing it with the sorter and verifying the EV size distribution with the nanoparticle tracking analysis technique.
Due to the increasing importance of EVs, having a pure population to study and characterize is becoming crucial. Here, we demonstrate a winner strategy to set up sorting to achieve this goal.
Introduction
Extracellular Vesicles (EVs) are a heterogeneous group of membrane-structured vesicles released by almost all cells, delimited by a lipid bilayer, unable to replicate on their own1. They can be found in several biofluids such as blood plasma, serum, saliva, breast milk, urine, bronchial lavage fluid, amniotic fluid, cerebrospinal fluid, and malignant ascites2. One of the main functions of EVs is to transport various molecules, including nucleic acids, proteins, lipids, and carbohydrates, between a donor and a recipient cell. This can occur through various mechanisms, such as direct membrane fusion, receptor-ligand interaction, endocytosis, and phagocytosis3,4. For this reason, they have been demonstrated to play an important role in a lot of physiological and pathological processes, and they show considerable promise as novel biomarkers of disease, as drug delivery vehicles, and as therapeutic agents5,6.
Mesenchymal stromal cells (MSCs) are multipotent cells that can be isolated from many tissues, including adipose tissue, dental pulp, umbilical cord blood, placenta, amniotic fluid, Wharton's jelly, and even the brain, lung, thymus, pancreas, spleen, liver, and kidney. In recent years, they have attracted considerable interest in regenerative medicine7. Adipose-derived mesenchymal stem cells (ASCs) can be harvested from fat tissue through a less invasive procedure compared to other sources like bone marrow, resulting in lower risks of severe complications and avoiding ethical issues8.
Additionally, adipose tissue contains a significantly higher concentration of MSCs than bone marrow (1% versus >0.01%) and other sources such as the dermis, dental pulp, umbilical cord, and placenta. MSCs are crucial in the regeneration of injured tissues and cells due to their differentiation ability and their secretion of a broad repertoire of growth factors, chemokines, and cytokines; these therapeutic benefits are attributable to their differentiation ability but also to the fact that they secrete a broad repertoire of growth factors, chemokines, and cytokines. A striking example is given by MSCs' therapeutic potential for orthopedic conditions, with the term "Musculoskeletal Diseases" having the higher number of registered clinical studies under clinicaltrials.gov (accessed 13th May 2024).
Moreover, MSCs can also secrete EVs that take part in tissue regeneration via transferring information to damaged cells or tissue and exert biological activity similar to the mother cells9,10. For this reason, MSC-EVs may be a valuable substitute for cell therapy to achieve a cell-free approach11, with two clinical studies involving MSC-EVs for orthopedic conditions (NCT05261360 and NCT04998058). However, several challenges still exist for the clinical applications of EVs. For example, there are some concerns about EV isolation techniques: most of them do not guarantee vesicle purity or integrity. Moreover, some isolation techniques are complex, time-consuming, and have low repeatability, making them unsuitable for clinical use12.
Cell sorting, on the other hand, is a commonly used method that allows for the isolation of single cells from heterogeneous cell suspensions by using specific fluorescent markers13. It can be used for many applications and adapted to different sample types. However, although cell sorting is a well-established and widely used technology, EV sorting is still very challenging because most EVs are below the minimum detection threshold for even the most sensitive flow cytometers. There are some features that make a sorter more suitable for this purpose. First of all, using a Jet-in-air system in which the stream suspending the particles is interrogated by lasers in air, rather than within a flow cell; this system preserves the sample by decreasing the stress to which it is subjected. A second important point is the presence of an "obscuration" bar between the stream and the collection lens that decrease the background optical noise of the instrument. Although it is low, the background noise is not completely eliminated and constitutes a reference that provides a partial window into the events that fall under the threshold: it is very important for the analysis of events that are close to the "limit of detection" of the instrument14. Finally, the sorter features a dual-path Forward Scatter (FSC) with two different masks that allow for improved discrimination between small and large particles in the sample.
Based on this, we developed a protocol aimed to separate carboxyfluorescein succinimidyl ester (CFSE) labeled MSC-EVs by using a high-sensitivity cell sorter. To minimize the manipulation of EVs and preserve their integrity and quantity, we avoided ultracentrifugation steps during the sample preparation. Furthermore, sorting conditions were adjusted to minimize stress on the vesicles, including further optimization of our instrument by reducing the sorting pressure associated with the nozzle size (70 μm nozzle for a pressure of 35 psi).
Protocol
The protocol here consists of four parts: (1) Sample preparation, (2) ASC-EVs characterization, (3) ASC-EVs sorting, and (4) Post sorting analysis. A schematic representing the workflow is shown in Figure 1.
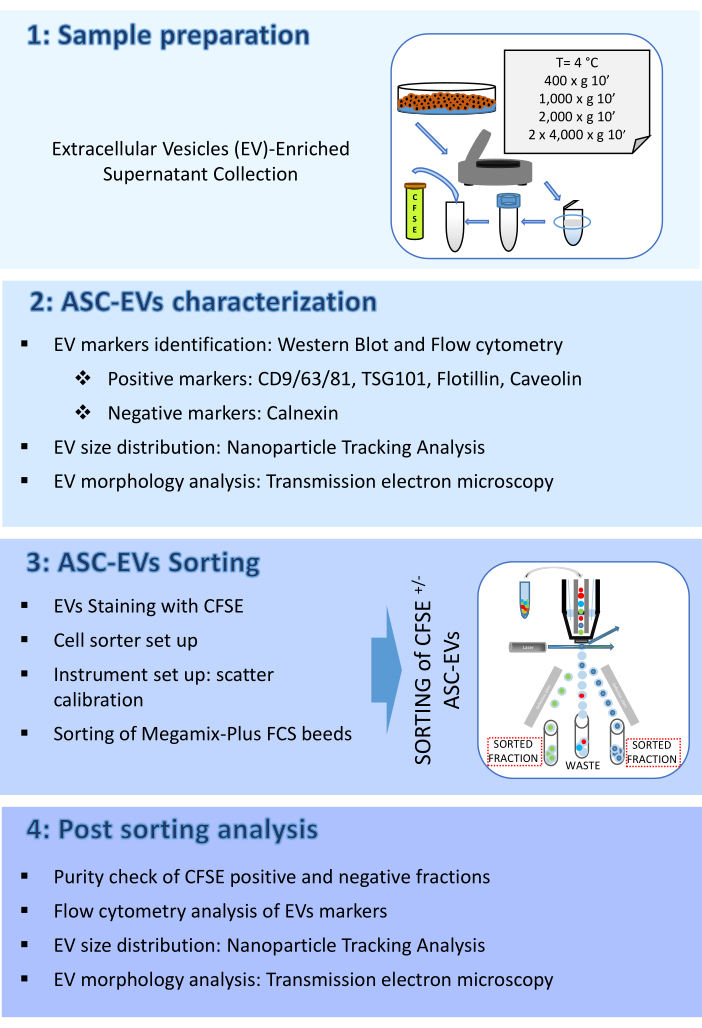
Figure 1: Protocol Flow chart. The flow chart shows the steps involved in the protocol. (1) sample preparation, (2) characterization of vesicles before sorting, (3) sorting, and (4) analysis of vesicles post-sorting. Please click here to view a larger version of this figure.
1. Sample preparation
- Extracellular vesicles (EV)-enriched supernatant collection
- Thaw or collect adipose-MSCs (ASCs) that were cultured the passage before EVs collection (usually passage 1 to 5) and seed per each ASC isolate an identical amount of cells (e.g., 1 million cells per 175 cm2 of flask surface results in approximately 60%-70% confluence).
- Grow ASCs in the appropriate culture medium (DMEM-F12 for this protocol) supplemented with EV-free fetal bovine serum (FBS) or human platelet lysate (hPL), as per the requested protocol, for 48-72 h.
- To obtain EV-free FBS or hPL, ultracentrifuge at 120,000 x g over night at 4 °C and use the supernatant.
NOTE: From this point on, indicated centrifugal force is always meant as average for the used instrument, rotor and tubes. Keep in mind that different rotors or tubes may have variable g-force per rpm and k-factor. For an easy way to compare rotors and adjust g-force and speed, refer to one of the available conversion tables https://www.beckman.it/centrifuges/rotors/calculator.
- To obtain EV-free FBS or hPL, ultracentrifuge at 120,000 x g over night at 4 °C and use the supernatant.
- At 90% cell confluence, detach and count ASCs and suspend in a proper volume of serum-free medium, ideally 1 mL per 1 x 106 ASCs. Seed ASCs in suspension in 24-well plates with 1 mL per well. Without serum, cells will remain in suspension and form a spheroid. After 96 h, collect the supernatant.
- Remove floating cells and debris by serial centrifugation at 4 °C: 400 x g for 10 min, 1,000 x g for 10 min, 2,000 x g for 10 min, and twice 4,000 x g for 10 min.
- Filter the supernatant through a 0.22 µm filter to remove remaining particles larger than 220 nm.
NOTE: Use immediately for downstream applications, or store for a maximum of one night at 4 °C or freeze at -80 °C if further steps are not performed within 24 h.
- EVs staining for sorting
- Prepare a 5 mM solution of 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE). Use it freshly prepared or freeze at -20 °C protected from light.
- Proceed directly from step 1.1.1-1.1.5 (within 24 h) or thaw -80 °C stored EVs-containing supernatants at 4 °C.
- Before staining, warm supernatants at 37 °C and add CFSE for a final concentration of 10 µM (500-fold dilution). Incubate for 1 h at 37 °C in the dark.
- During incubation, add 2 mL of PBS to a centrifugal concentrator (regenerated cellulose membrane, MWCO 100 kDa), cap, and centrifuge at 4,000 x g for 10 min in a swinging bucket rotor. Remove the unfiltered PBS from the bottom of the filter device and aspirate the filtrate from the collection tube.
NOTE: The centrifugal ultrafiltration protocol outlined is based on processing up to 15 mL of the sample (maximum volume). - Add up to 15 mL sample to the centrifugal concentrator and cap the device. Centrifuge at 4,000 x g for up to 30 min.
NOTE: This will yield a final sample of 500 µL on average. However, depending on factors such as the nature of the sample and flow rate, the centrifugation time required to achieve the desired concentration can vary. - Remove the device from the centrifuge and empty the collection tube. Add 14 mL of PBS to the filter device. Centrifuge at 4,000 x g for up to 30 min.
- Repeat step 1.2.6.
- Recover concentrated CFSE-stained EVs sample from the filter device, approximately 500 µL, and store 100 µL at 4 °C in the dark while proceeding with the sorting of the remaining sample.
2. ASC-EVs characterization
- Western blot analysis of EV markers
- Pellet ASCs (1 x 106) at 376 x g at RT for 5 min and suspend in appropriate lysis buffer supplemented with protease inhibitors. Perform protein quantification with the assay of choice.
NOTE: As experienced in this study, the best quantification results are obtained with the Bicinchoninic acid assay technique. - Quantify ASCs-concentrated supernatant equivalent to 1 x 106 ASCs with Bradford technique or pellet EVs corresponding to 1 x 106 ASCs at 100,000 x g at 4 °C for 1 h and suspend in appropriate lysis buffer supplemented with protease inhibitors. Perform protein quantification with the assay of choice.
NOTE: As experienced in this study, the best quantification results for EV pellets are obtained with the Bicinchoninic acid assay technique. - If concentrated supernatant in place of purified EVs is used, lyse the samples in 5% 2-Mercaptoethanol and 2x Laemmli buffer.
NOTE: As experienced in this study, both concentrated supernatant and purified EVs give comparable results for positive and negative EV markers. - Load and separate samples (1-10 µg) in a 10% polyacrylamide gel at 110 V for 90 min.
- Transfer to a nitrocellulose membrane at 250 mA for 120 min.
- Stain membrane with Ponceau S to visualize samples and ladder transfer. Remove Ponceau S with PBS under gentle shaking.
- Block membranes with 5% non-fat dried milk and 0.1% Tween in PBS for 60 min.
- Probe membranes with appropriate antibodies at working dilutions, including positive (e.g., in this work CD9, CD81, TSG101, and Flotillin) and negative (e.g., in this work Calnexin) EV markers, at 4 °C overnight.
- Wash with 0.1% Tween in PBS and incubate with appropriate peroxidase-conjugated secondary antibodies for 45 min at RT before band reveals with ECL system of choice. Acquire images with an available imaging system (Figure 2A).
- Pellet ASCs (1 x 106) at 376 x g at RT for 5 min and suspend in appropriate lysis buffer supplemented with protease inhibitors. Perform protein quantification with the assay of choice.
- Flow cytometry analysis of EV markers: surface staining
- Before use, centrifuge each monoclonal antibody (mAb) at 15,000-17,000 x g for 30 min at 4 °C to eliminate aggregates, which can cause false positive signals. Further, filter mAbs into separate 0.22 µm centrifugal filter tubes at 1,000 x g at 4 °C until all the mixture has passed through the filter and no antibody liquid remains on the surface of the filter. Store mAbs at 4 °C.
- Prepare a 1:10 dilution of CFSE-stained EVs (see step 1.2.1-1.2.8) in 0.22 µm-filtered PBS.
- Incubate 100 µL of samples, or 0.22 µm-filtered PBS, with or without EV-specific mAbs (anti-CD9/63/81) or MSC-specific mAbs (anti-CD44), previously titrated. Perform incubation at 4 °C in the dark for 30 min.
NOTE: This protocol, developed to detect typical EVs and MSC-lineage markers, may be extended to all other cell lineage or EVs subtype-specific surface markers. Do not use fluorochromes falling in the fluorescein isothiocyanate (FITC)channel to avoid overlap with CFSE staining of EVs. Perform a single staining by using each mAb conjugated with one fluorochrome, for example, allophycocyanin (APC). Multicolor staining is possible, but controls must be included to address potential antibody steric hindrance issues. It is necessary to test the individual antibodies alone or within a mix and ensure that the signal is comparable. Additionally, FMOs need to be included among the controls. - Set up scatter calibration as described in step 3.2.
- Create a dot plot of the side scatter (SSC) log scale versus the FITC log scale and run a tube of 0.22 µm-filtered either PBS or unstained EVs; set the threshold on the FITC channel and adjust it to the highest values that exclude the majority of background noise. Draw a region identifying the CFSE positive events.
- Create an APC histogram plot gated on CFSE positive events region and run a tube of 0.22 µm-filtered either PBS or unstained EVs.
NOTE: For APC gain, use the gain established with the instrument QC, although it is recommended to perform a specific gaintration in order to optimize the instrument performances. - Add 200 µL of 0.22 µm-filtered PBS to stained samples and acquire. Use a low flow rate (10 µL per min) for acquisition and recording. If possible, record at least 5,000 events in the FITC positive gate.
- Use the mAb-stained PBS control to detect possible aspecific signals of the mAbs. Read all sample tubes at the same flow rate to ensure consistency between runs.
- To avoid sample cross-contamination, run a detergent for 10 s between each analyzed tube, followed by 10 s with deionized (DI) water (Figure 2B).
NOTE: To avoid any fluidics instrument instability, acquire the sample for 10 s, and then start recording.
- Flow cytometry analysis of EV marker: Intracellular staining
NOTE: For intracellular staining, use a specific kit containing fixation and permeabilization reagents. Anti-Caveolin mAb is conjugated with Alexa 488. The IC staining has been performed in the absence of CFSE.- Before use, centrifuge the mAb as previously described (step 2.2.1).
- For intracellular staining, follow the manufacturer's instructions (Figure 2C).
- Characterization of EV concentration and size by nanoparticle tracking analysis (NTA)
- Make appropriate dilutions of the samples to obtain 20-120 events in the viewing frame of the NTA instrument display.
- Inject the samples into the NTA instrument sample chamber using a 1 mL insulin syringe.
- Set the NTA software for recording as follows: 5 standard measurements, 60 s each, and a flow pump set at 30.
- Set the light intensity to an appropriate value allowing to clearly distinguish particles from the background and run the capture script on the NTA software.
NOTE: Camera level setting may influence particle detection. If the value is too high, a milky background and strong particle light scattering emerge, covering most of the particle signals. If the value is too low, although a darker background is obtained, it is possible most of the less bright events are missed. Each sample should be monitored for optimal setting. - Adjust the dilution factor if particles per frame are lower than 20 or more than 80.
- Set the threshold to 4 to analyze the average modal size and particle concentration of the supernatant. Take into account initial dilution factors.
- Clean the instrument chamber with deionized water after each sample to avoid cross-contamination.
- Open the NTA analysis software and perform analysis on acquired samples, taking into account the dilution factor (Figure 2D).
- Characterization of EV morphology by transmission electron microscopy (TEM)
- Collect ASCs-concentrated supernatant or isolate EVs by ultracentrifugation (100,000 x g at 4 °C for 1 h) followed by pellet suspension in the same volume of PBS.
- Allow 5 μL of the concentrated supernatant or EVs to be absorbed on carbon-coated grids for 10 min. Blot excess drops of liquid with filter paper.
- Perform negative stain with 2% uranyl acetate in aqueous suspension for 10 min using an identical volume of the sample drop (5 µL). Remove the excess uranyl acetate by touching the grid with filter paper.
- Dry the grid at room temperature (RT) and examine it with a transmission electron microscope at 120 kV (Figure 2E).
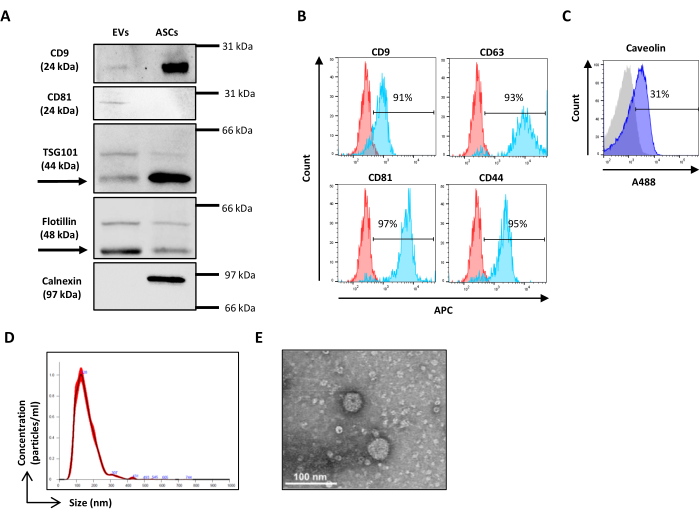
Figure 2: ASC-EVs characterization. (A) Representative Western blot of EVs positive (CD9, CD81, TSG101, and Flotillin) and negative (Calnexin) markers. Corresponding molecular weights are reported, and ASCs lysates have been used as control. (B) Flow cytometry analysis of EVs markers. The expression of the following markers was analyzed: CD9, CD63, CD81 and CD44. Only CFSE-positive ASC-EVs were analyzed for marker expression. Histograms represent unstained (red histograms) and stained (blue histograms) ASC-EVs.(C) Flow cytometry intracellular analysis of EVs marker Caveolin. Histograms represent unstained (grey histogram) and stained (blue histograms) ASC-EVs.(D) Characterization of ASC-EVs by NTA. Histograms represent the concentration (particles/mL)/size (nm) of the sample. (E) Visualization of ASC-EVs by TEM. Scale bars = 100 nm. Please click here to view a larger version of this figure.
3. ASC-EVs sorting
- Cell sorter set up
NOTE: A cell sorter is a flow cytometer that enables the isolation of a pure population from a heterogeneous starting sample. Cell sorter separates the target particles by oscillating the stream with a piezoelectric at a high frequency to generate drops containing an event. The drops that contained the particles of interest, such as cells or vesicles, are charged and deflected through metal deflection plates. The sorted fraction is used to perform downstream analysis.- Open the pressure line and vacuum.
NOTE: Some sorters have an automatic startup, while others have a manual startup. A sorter with a manual startup is preferred, as it allows the optimization of some technical features such as frequency, pressure, and the choice of the nozzle tip. In particular, it is recommended to work with a frequency of 66,000 Hz, a pressure of 35 psi, and a 70 µm nozzle. - Switch on the instrument, open the biosafety cabinet, and launch the sorter software.
- Pressurize the fluidics and then switch on the fluidics system.
- Activate Drop Drive, the piezoelectric crystal in the nozzle that vibrates to have droplets.
- Perform Debubbling procedure to eliminate the presence of bubbles in the system and then run a 5 mL tube of cleaning solution for 5 min at high differential pressure and 5 mL tube of DI water for 5 min.
- Perform manual Startup procedure.
- On the Laser Control Tab, press the Laser Power button to turn the laser power on.
NOTE: Repeat this procedure for all the lasers: Blue 488 nm, Yellow-Green 561 nm, Violet 405 nm, and Red 640 nm. - Examine the vertical alignment of the stream.
NOTE: In case of need, move the nozzle assembly stage, in particular, front and back micrometer, left and right micrometer, and gimbal to adjust the vertical alignment and verify the vertical stream with up and down micrometer; for the best alignment, the stream must be vertical on each laser. If this is the first time one is using a specific nozzle, a Laser Delay needs to be done. On the touch screen panel, on the Laser and Stream Intercept tab, select the correct nozzle size, press the Laser Delay button, and follow the laser delay instructions to perform the laser delay procedure. In general, when changing sheath pressure and, consequently, nozzle tip, the Laser Delay Determination is executed prior to finding alignment. At the end of the Laser Delay procedure, it is suggested that the Background Image Subtraction Procedure be performed following the instructions on the screen in order to minimize the background signal. - Perform Laser Spot Determination by pressing on the Laser Stream Intercept tab, the Green Arrow button, and following the instructions on the touch screen monitor.
- On the Laser Control Tab, press the Laser Power button to turn the laser power on.
- Initialize Intellisort. Set the drop drive frequency and a default amplitude value, which causes the stream to form a droplet.
- Once this step has been performed, load a saved configuration. To load a saved setting configuration, select Load Sort Setting on the sorter software toolbar. After checking different frequencies and amplitudes, choose the best one that ensures the best drop stability. Work at a frequency of 66,000 Hz and an amplitude of around 40-45 V.
- Perform Fine Laser Alignment procedure.
- Load in the sampler a 5 mL tube of QC alignment beads and run it; select on the Fine Alignment tab the desired parameter on the X and Y axis in order to visualize beads well compacted and collimated. On the Data Display Area, select first of all 488 - 513/26-H for the Y-axis parameter and 488-FSC1-H for the X-axis parameter. Then, to fine tune all lasers, select 405-488/59-H for the Y-axis parameter and 640-795/70-H for the X-axis parameter.
NOTE: The parameters selected depend on the laser configuration of the sorter. The instrument manual suggestion is the choice of the lasers that have the greatest spatial separation on the pinhole strip. For this purpose, use a Violet laser (405 nm) and a Red laser (640 nm). - Once the manual alignment is checked by the sorter specialist, perform the Automatic QC. Select the diameter of the QC beads: 3 µm diameter.
NOTE: At the end of this procedure, the result could be QC did not pass or QC passed. In case the QC fails, it is necessary to optimize manually the alignment. - Save the QC acquisition on the software Alignment protocol.
- Load in the sampler a 5 mL tube of QC alignment beads and run it; select on the Fine Alignment tab the desired parameter on the X and Y axis in order to visualize beads well compacted and collimated. On the Data Display Area, select first of all 488 - 513/26-H for the Y-axis parameter and 488-FSC1-H for the X-axis parameter. Then, to fine tune all lasers, select 405-488/59-H for the Y-axis parameter and 640-795/70-H for the X-axis parameter.
- Finalize Intellisort procedure
- Locate the deflection plates in the correct position in the sorter and turn on the voltage (3,000 V charge on deflection plates is recommended).
- Choose 6 Tube Holder sort output, enable the streams selecting them on the Stream Indicator, and perform the Test Stream.
NOTE: If the streams are not clearly separated and defined, adjust the charge phase, defanning, or modify other parameters in order to obtain a clearly defined and not oscillating stream. - Enable the Intellisort Automatic Drop Delay Determination button. This step allows for setting the correct drop delay. After this step, verify the streams again.
- Activate Intellisort Maintain Mode. Verify the Drop Delay manually.
- Load the manual Drop Delay protocol on sorter software. Acquire fluorescent control beads.
- Insert the correct slide holder. In the sorter software, select Sort > Drop Delay Wizard. Select Sort Logic.
- Verify with a fluorescent microscope the presence of 97% of fluorescent beads on the fifth puddle.
- Open the pressure line and vacuum.
- Instrument setup: Scatter calibration
NOTE: Scatter calibration is performed by using Verity Shells beads (henceforth referred to as hollow organo silica beads). These organo silica beads are characterized by a refractive index distribution and light scattering properties closer to EVs than polystyrene beads, thus allowing the setup of the SSC and FSC voltage to better discriminate between EV-like events and the electronic noise. The organo silica beads are constituted by two vials with bead mixtures, VER01A (189 nm beads) and VER01B (374 nm beads). Each vial contains a mixture of hollow organo silica beads and 380 nm green fluorescent beads, referred to as reference beads. The concentration of the hollow organo silica beads is 1 x 108 beads/mL.- Prepare a sample of VER01A and VER01B beads by diluting one drop (50 μL) of each in 1 mL of 0.22 µm-filtered PBS with a final concentration of 5,000 beads/µL.
NOTE: Vortex the beads for 5 s before using. - Create a dot plot of the SSC log scale versus the FITC log scale.
- Acquire VER01B beads and adjust the voltage to discriminate the reference beads well in the upper right part of the SSC/FITC dot plot (Figure 3A).
- Set a region around this population of beads.
- Create a dot plot of the SSC log scale versus the FSC log scale. Identify the reference beads and the VER01B beads population. Set a region around the VER01B beads population (Figure 3B).
- Acquire VER01A beads and create a region around this beads population (Figure 3C).
NOTE: This population of beads partly overlaps with the background noise of the instrument. - Acquire 0.22 µm-filtered PBS and adjust the threshold to decrease the background noise of the instrument without losing the visualization of the smallest beads.
NOTE: Scatter calibration can be done using FSC Megamix beads (henceforth referred to as FSC polystyrene beads), a mix of fluorescent beads with the following diameters: 100 nm, 300 nm, 500 nm, and 900 nm. The use of the hollow organo silica beads has to be preferred to FSC polystyrene beads because they have refractive index distribution and light scattering properties similar to EVs. The population of 300 nm FSC polystyrene beads overlaps with the reference of the hollow organo silica beads. Therefore, a gate based on hollow organo silica beads selects similar-sized EVs on the flow cytometer.
- Prepare a sample of VER01A and VER01B beads by diluting one drop (50 μL) of each in 1 mL of 0.22 µm-filtered PBS with a final concentration of 5,000 beads/µL.
- Sample acquisition
- Create a dot plot of the SSC log scale versus the FITC log scale, acquire 0.22 µm-filtered PBS, and define the reference background noise (Figure 4A).
NOTE: Set the cytometer's flow rate to Low and control the flow rate. Number of events/s must not be more than 2,000 events/s. Flow rate Low means at low differential pressure. - Acquire the control sample (unstained EVs) and check the flow rate. Ensure that the number of events/s is around 5,000 events/s.
NOTE: If the number of events exceeds that suggested, dilute the sample. - Acquire PBS stained and treated as the CFSE sample and check that no positive events are visible (Figure 4B).
- Acquire the CFSE stained sample. Dilute it based on the flow rate. Draw a region identifying the CFSE positive events and one identifying the CFSE negative events. Use an unstained sample as a control (Figure 5A, B). These are the sorting regions.
- Create a dot plot of the SSC log scale versus the FITC log scale, acquire 0.22 µm-filtered PBS, and define the reference background noise (Figure 4A).
- Sample sorting
- On the sorter software, open a new protocol (on the toolbar select File > Protocol > New).
- On the sorter software, click Data Acquisition Setting; on the window Acquisition Parameter, select the channels of interest and disable the others. For this specific panel, the signal abled are: 488-FSC1, 488-FSC2, 488-SSC, and 488-513/26 (for CFSE signal).
- Locate on the sampler 5 mL tube of sample. On the touch screen, click the Load button, and on the sorter software toolbar, select Acquisition > Start (or press the F2 button). A window of sample properties will appear; insert the name of the sample and click ok.
- Create the panel, with the plot of interest and create the gating strategy.
- On the sorter software toolbar, select Histogram > Create histogram.
- In the workspace, create three dot plots, the first one with on x-axis 488-FSC1 Height-Log parameter and y-axis 488-SSC Height-Log, the second one with on x-axis 488-FSC2 Height-Log parameter and y-axis 488-SSC Height-Log, the third one with on x-axis 488-513/26 CFSE Height-Log parameter and y-axis 488-SSC Height-Log.
- On the Sort Tab, identify the region/s to be sorted. On sorter software, click Sort Setting. On the Sort Logic and Statistic window, select the region to be sorted in the logic builder.
NOTE: To make a sort decision, there are different settings to take into account: Sort Logic, Sort Mode, and Drop Envelope. The Sort Logic is referring to the gating strategy. The events that are outside the selected gate are not sorted. The Sort Mode is related to the final output. Work in a Purify Mode, in this way, all the drops sorted contain only the positive events desired. The Drop Envelope defines how many drops will be charged and sorted related to the location in the drop of the positive event: choose 1-2 drops in order to be sure that all the positive events are sorted. - When the flow rate is stable, stop acquisition (on the toolbar of the sorter software, select Acquisition > Stop or press the F2 button again). Start sorting (on the toolbar of sorter software, select Sort > Start or press the F4 button).
NOTE: Before starting with the sorting, check the Test Stream. Verify that the sorted fraction falls correctly into the collection tube. - Save the sort setting. On the toolbar of the sorter software, select Sort > Save Sort Settings.
- Purity check of sorted population
- Wash the instrument by acquiring a 5 mL tube of cleaning solution for 10 min at high differential pressure and a 5 mL tube of DI water for 10 min.
- Acquire 0.22 µm-filtered PBS and check that no CFSE-positive events are present.
- Dilute 5 μL of the sorted sample in 100 µL of 0.22 µm-filtered PBS.
- Acquire and record all the sample volumes (Figure 5C, D).
NOTE: Re-analysis of both CFSE positive and negative sorted EVs are reported.
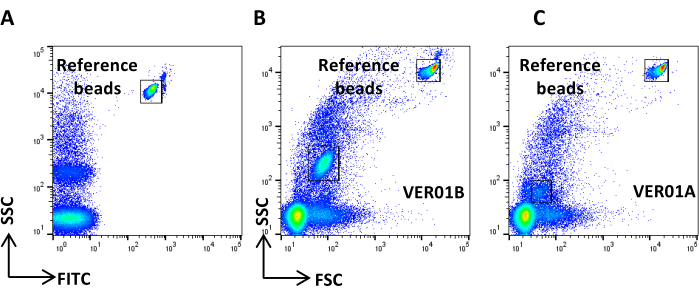
Figure 3: Physical parameter setting with hollow organo silica beads. (A) SSC/FITC dot plot: reference green, fluorescent beads have been used to set SSC parameter. SSC/FSC dot plot of (B) VER01B and (C) VER01A beads. Please click here to view a larger version of this figure.
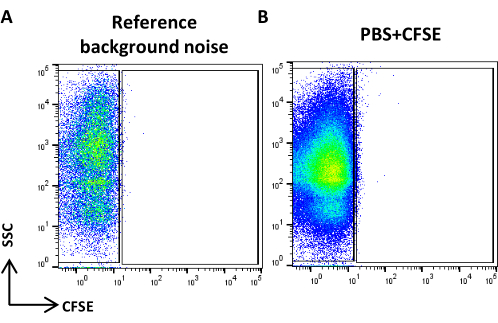
Figure 4: Reference background noise. (A) SSC/CFSE dot plot of PBS sample. (B) SSC/CFSE dot plot of PBS + CFSE sample. Please click here to view a larger version of this figure.
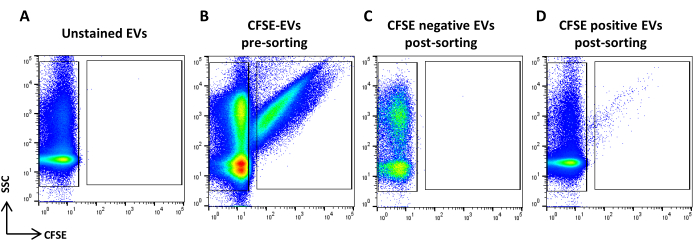
Figure 5: CFSE stained ASC-EVs sorting. (A) SSC/CFSE dot plot of unstained EVs, (B) CFSE stained EVs, (C) CFSE negative EVs post-sorting, and (D) CFSE positive EVs post-sorting. Please click here to view a larger version of this figure.
4. Post sorting analyses
NOTE: Due to the limited amount of material after sorting, it may not be possible to perform all analyses. With the amount obtained, the following are performed.
- Characterization of EV by flow cytometry: surface staining (see step 2.2)
NOTE: As previously described for pre-sorting samples except for sample preparation. Samples after sorting may be used undiluted.- Use undiluted sorted samples and proceed as per step 2.2.3. (Figure 6A)
- Characterization of EV concentration and size by NTA (see step 2.4)
NOTE: As previously described for pre-sorting samples except for sample preparation. Samples after sorting may be used undiluted.- Use undiluted sorted samples and proceed as per step 2.4.2. (Figure 6B)
- Characterization of EV morphology by TEM (see step 2.5)
NOTE: As previously described for pre-sorting samples except for sample preparation.- Add 2 mL of PBS to a centrifugal concentrator (regenerated cellulose membrane, MWCO 100 kDa), cap, and centrifuge at 4,000 x g for 10 min in a swinging bucket rotor. Remove unfiltered PBS from the bottom of the filter device.
- Aspirate filtrate from the collection tube. The centrifugal ultrafiltration protocol outlined is based on processing up to 15 mL samples (maximum volume).
- Add up to 15 mL sample to the AU-15 filter and cap the device. Centrifuge at 4,000 x g for up to 30 min. Concentrate up to the minimal volume the device allows, approximately 100-150 µL.
- Recover the concentrated sorted EV sample from the filter device and proceed as previously described for pre-sorting samples TEM (step 2.5.2) ( Figure 6C).
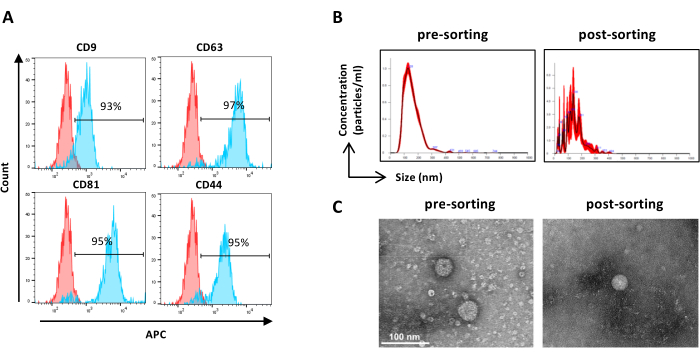
Figure 6: Characterization of sorted ASC-EVs. Flow cytometry analysis of EV markers. The expression of the following markers was analyzed: CD9, CD63, CD81, and CD44. Only CFSE-positive ASC-EVs were analyzed for marker expression. (A) Histograms represent unstained (red histograms) and stained (blue histograms) ASC-EVs. (B) Characterization of ASC-EVs by NTA. Histograms represent the concentration (particles/mL)/size (nm) of the pre-sorting (left) and post-sorting (right) samples. (C) Visualization of ASC-EVs by TEM of pre-sorting (left) and post-sorting (right) sample. Scale bars = 100 nm. Please click here to view a larger version of this figure.
Results
The FSC polystyrene beads have been sorted to validate the instrument setup and sorting conditions. FSC polystyrene beads are a mix of fluorescent beads ranging from 100 nm, 300 nm, 500 nm, and 900 nm and are visible on the FITC channel. Figure 7A shows the SSC log scale versus the FITC log scale dot plot with the four populations of beads before sorting. The fluorescent populations of 100 nm, 300 nm, and 500 nm were gated and sorted. Sorted beads were analyzed for purity and enrichment as a...
Discussion
Analyzing and sorting EVs is challenging due to their small size and the fact that they are near the detection limit of most flow cytometers. Our objective was to develop a protocol for isolating EVs derived from AMSCs labeled with CFSE. CFSE was selected as the staining method due to its reported high EVs labeling efficiency (≥90%), without the formation of unwanted particles such as protein aggregates given by antibodies. Nevertheless, it is possible that few EVs without esterase could be missed and future studie...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Emanuele Canonico for technical support. Part of this work was carried out in ALEMBIC, an advanced microscopy laboratory established by IRCCS Ospedale San Raffaele and Università Vita-Salute San Raffaele. The work of Enrico Ragni and Laura de Girolamo was supported by the Italian Ministry of Health, "Ricerca Corrente".
Materials
| Name | Company | Catalog Number | Comments |
| 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester | Merck | 150347-59-4 | |
| Adipose Mesenchymal Stromal Cells | Wepredic, Parc d'affaires, 35760 Saint-Grégoire, France | Cells used in this study | |
| Alexa 488 anti-Caveolin | R&D Systems | IC5736G | Flow cytometry antibody |
| APC anti-human CD44 | BioLegend | 338805 | Flow cytometry antibody |
| APC anti-human CD63 | BioLegend | 353007 | Flow cytometry antibody |
| APC anti-human CD81 (TAPA-1) | BioLegend | 349509 | Flow cytometry antibody |
| APC anti-human CD9 | BioLegend | 312107 | Flow cytometry antibody |
| BC CytoFLEX S | Beckman Coulter | BC CytoFLEX S equipped with 3 lasers, Blue, Red and Violet | |
| Flow-Check Pro Fluorospheres | Beckman Coulter | A63493 | Fluorescent control beads for MoFLO Astrios EQ |
| FlowJo software (version 10.8.1) | BD | version 10.8.1 | Analysis software |
| IntraSure kit | BD Biosciences | 641776 | Fixation and permeabilization for intracellular staining |
| Megamix-Plus FSC | BioCytex | 7802 | FSC polystyrene beads |
| MoFLO Astrios EQ | Beckman Coulter | MoFLO Astrios EQ equipped with 4 lasers, Blue, Yellow - Green, Violet and Red | |
| Mouse anti-FLOT1 antibody | BD Transduction Laboratories | 610820 | Western Blot antibody |
| NanoSight NS300 | Malvern | NS300 | |
| Rabbit anti-Calnexin antibody | Origene | TA336279 | Western Blot antibody |
| Rabbit anti-CD9 and CD81 antibody (ExoAb antibody kit) | System Biosciences | EXOAB-KIT-1 | Western Blot antibodies |
| Rabbit anti-TSG101 antibody | Merck | HPA006161 | Western Blot antibody |
| Triton X-100 | Merck | 9036-19-5 | |
| Ultra Rainbow Fluorescent Particles | Spherotech | URFP-30-2 | |
| Ultracel 100 kDa MWCO | Merck | UFC910024 | |
| VER01 - Verity Shells | Exometry | Organo silica beads for scatter calibration |
References
- Welsh, J. A., et al. Minimal information for studies of extracellular vesicles (misev2023): From basic to advanced approaches. J Extracell Vesicles. 13 (2), e12404 (2024).
- Xu, R., Greening, D. W., Zhu, H. J., Takahashi, N., Simpson, R. J. Extracellular vesicle isolation and characterization: Toward clinical application. J Clin Invest. 126 (4), 1152-1162 (2016).
- Dixson, A. C., Dawson, T. R., Di Vizio, D., Weaver, A. M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. 24 (7), 454-476 (2023).
- Witwer, K. W., Wolfram, J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 6 (2), 103-106 (2021).
- Cheng, L., Hill, A. F. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 21 (5), 379-399 (2022).
- Du, S., et al. Extracellular vesicles: A rising star for therapeutics and drug delivery. J Nanobiotechnology. 21 (1), 231 (2023).
- Keshtkar, S., Azarpira, N., Ghahremani, M. H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res Ther. 9 (1), 63 (2018).
- Orbay, H., Tobita, M., Mizuno, H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012, 461718 (2012).
- Caplan, A. I., Dennis, J. E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 98 (5), 1076-1084 (2006).
- Rani, S., Ryan, A. E., Griffin, M. D., Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther. 23 (5), 812-823 (2015).
- Galipeau, J., Sensébé, L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell. 22 (6), 824-833 (2018).
- Jia, Y., et al. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics. 12 (15), 6548-6575 (2022).
- Ohnuma, K., Yomo, T., Asashima, M., Kaneko, K. Sorting of cells of the same size, shape, and cell cycle stage for a single cell level assay without staining. BMC Cell Biol. 7, 25 (2006).
- Morales-Kastresana, A., et al. High-fidelity detection and sorting of nanoscale vesicles in viral disease and cancer. J Extracell Vesicles. 8 (1), 1597603 (2019).
- Mortati, L., et al. In vitro study of extracellular vesicles migration in cartilage-derived osteoarthritis samples using real-time quantitative multimodal nonlinear optics imaging. Pharmaceutics. 12 (8), 734 (2020).
- Andreu, Z., Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 5, 442 (2014).
- Mildmay-White, A., Khan, W. Cell surface markers on adipose-derived stem cells: A systematic review. Curr Stem Cell Res Ther. 12 (6), 484-492 (2017).
- Welsh, J. A., Tang, V. A., Van Der Pol, E., Gorgens, A. MIFlowCyt-EV: The next chapter in the reporting and reliability of single extracellular vesicle flow cytometry experiments. Cytometry A. 99 (4), 365-368 (2021).
- Maia, J., et al. Employing flow cytometry to extracellular vesicles sample microvolume analysis and quality control. Front Cell Dev Biol. 8, 593750 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved