A subscription to JoVE is required to view this content. Sign in or start your free trial.
Detection of Regulated Ergot Alkaloids in Food Matrices by Liquid Chromatography-Trapped Ion Mobility Spectrometry-Time-of-Flight Mass Spectrometry
In This Article
Summary
This protocol presents a validated liquid chromatography-ion mobility-high resolution mass spectrometry method to determine the presence of ergot alkaloids in food in compliance with the recently released Commission Regulation (EU) 2023/915.
Abstract
Ion mobility mass spectrometry (IMS) acts as an additional separation dimension when integrated into liquid chromatography-mass spectrometry (LC-MS) workflows. LC-IMS-MS methods provide higher peak resolution, enhanced separation of isobaric and isomeric compounds, and improved signal-to-noise ratio (S/N) compared to traditional LC-MS methods. IMS provides another molecular characteristic for the identification of analytes, namely the collision cross section (CCS) parameter, reducing false positive results. Therefore, LC-IMS-MS methods address important analytical challenges in the field of food safety (i.e., detection of compounds at trace levels in complex food matrices and unambiguous identification of isobaric and isomeric molecules).
Ergot alkaloids (EAs) are a family of mycotoxins produced by fungi that attack a wide variety of grass species, including small grains such as rye, triticale, wheat, barley, millet, and oats. Maximum levels (MLs) of these mycotoxins have been established in several foodstuffs, as detailed in the Commission Regulation EC/2023/915. This new legislation includes six main EAs and their corresponding epimers, so an efficient methodology is required to properly distinguish these isomeric molecules considering their co-occurrence.
Therefore, the goal of this protocol is to show how the integration of IMS in LC-MS workflows contributes to the separation of isomeric EAs, enhancing the selectivity of the analytical method. Additionally, it illustrates how the generation of CCS libraries through the characterization of analytical standards provides higher confidence for the identification of mycotoxins. This protocol is designed to clearly explain the benefits of implementing IMS in food safety, taking as an example the determination of EAs in cereals. A QuEChERS-based extraction followed by an LC-trapped ion mobility spectrometry (TIMS)-MS analysis provided limits of quantification ranging from 0.65 to 2.6 ng/g with acceptable accuracy (although low recovery for ergotaminine) at 1.5x, 1x, and 0.5x the ML and exhibited a negligible matrix effect.
Introduction
Ion mobility mass spectrometry (IMS) is becoming a growingly used analytical technique, often presented as an additional separation dimension integrated into traditional liquid/gas chromatography (LC/GC) coupled to MS workflows. IMS consists of the separation of molecules along a mobility cell, filled with a buffer gas, under an electric field and at atmospheric pressure1. Depending on the mass-to-charge ratio (m/z) and the geometrical conformation, an ionized molecule will interact with the buffer gas as it moves across the mobility cell, which is reflected in the ion mobility (K) parameter2 and calculated through the following equation:
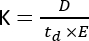
where D represents the total drift length, td is the total drift time, and E is the electric field. Therefore, K is measured in m2 V−1 s−1, although for practical reasons it is often expressed as cm2 V−1 s−1. The intrinsic capability to move across the mobility cell can be measured by the drift time and later converted to the so-called collision cross section (CCS) value, which is a highly reproducible parameter for each molecule independently of the IMS instrument3. The CCS can be derived from the mobility following this equation:
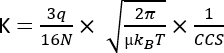
q being the charge of the ion; N the buffer gas number density; µ the reduced mass of the collision partners buffer gas-ion; kB the Boltzmann constant; and T the buffer gas temperature. Therefore, IMS provides additional information complementary to the analytical data resulting from chromatography and MS analyses.
The implementation of IMS in LC-MS platforms has been shown to increase the reliability of analytical determinations, especially when working with compounds that are at trace concentrations. Several studies have reported that LC-IMS-MS methods improve the quality of mass spectra by reducing background noise, which ultimately affects the sensitivity of the method, and reduces the rate of false positives and negatives provided by multi-residue LC-MS methodologies4,5,6. Further, the reproducibility of CCS values allows the comparison not only between different instruments using the same technology, but also between different ion mobility technologies, namely traveling wave ion mobility spectrometry (TWIMS), trapped ion mobility spectrometry (TIMS), and drift tube ion mobility spectrometry (DTIMS)2,7, which are the most frequently used systems1. Thus, a remarkable consequence of the potential of CCS as an identification parameter lies in the possibility of building CCS libraries, reflected in its applicability in metabolomics studies8. Nonetheless, one of the most powerful features of IMS is the ability to separate isomeric and isobaric compounds that may not be sufficiently resolved by LC-MS methods. This may be the case when working with large sets of analytes of interest in complex matrices, which is a common situation in environmental and food analysis. In this context, LC-IMS-MS methods have been proposed for the monitoring of pesticides and, to a lesser extent, veterinary drugs and mycotoxins in food9.
Due to their high resolving power and selectivity, LC/GC-IMS-MS platforms emerge as the most useful tools to address some of the current challenges in food safety, especially those related to isomeric mixtures. The health concern related to isomeric mixtures as food contaminants has been reflected in the current European legislation, which, for instance, limits the maximum concentration of six main ergot alkaloids (EAs) and their corresponding six epimers in several food products10.
EAs constitute a family of toxic secondary metabolites produced by a wide range of fungi, mainly of the family Clavicipitaceae (e.g., Claviceps purpurea, the most important EA producer due to its wide host range), but also Trichocomaceae, which can parasitize the seed head of living plants (such as rye, barley, wheat, and oat) at the time of flowering11,12. Under specific conditions, especially temperature and water activity, Claviceps fungi can produce EAs that accumulate in fruiting bodies, known as sclerotia or ergot, in the host crop. To a certain extent, EAs can withstand the processing of the raw material until reaching the final product; therefore, breaking into the food chain. Ingestion of contaminated food can lead to EA intoxication, known as ergotism, which presents with acute symptoms such as abdominal pain, vomiting, burning sensation of the skin, insomnia, and hallucinations13. To reduce the impact of EAs on human health, the European Commission set maximum levels (MLs) in several foods for the sum of the main EAs: the R-epimers ergometrine (Em), ergotamine (Et), ergosine (Es), ergocristine (Ecr), ergokryptine (Ekr), and ergocornine (Eco) and their corresponding S-epimers: ergometrinine (Emn), ergosinine (Esn), ergotaminine (Etn), ergocorninine (Econ), ergokryptinine (Ekrn), and ergocristinine (Ecrn). These compounds can epimerize from R to S forms and vice versa, especially under exposure to strong light, prolonged storage, or contact with some solvents at high or low pH 12. Although the proportion of R and S forms may vary under different conditions, the EFSA CONTAM Panel reported a higher occurrence of R forms than S forms after reviewing available literature on EAs in food products14. Hence, the MLs vary depending on several factors, such as the susceptibility of the crop, degree of processing, or frequency of consumption. In the EU framework, MLs for milled products of barley, wheat, spelt, and oat have been set at 50 or 150 µg/kg (depending on the ash content lower or higher than 900 mg/100 g, respectively), whereas cereals intended directly for human consumption are subject to an ML of 150 µg/kg, except for cereal-based baby food, in which the ML is reduced to 20 µg/kg10.
This stringent legislation requires analytical methodologies sensitive enough to determine trace concentration (µg/kg) levels while properly identifying regulated EAs and their corresponding epimers, as both forms, R- and S-isomers, can be found together in contaminated samples. This task represents a major challenge since each toxin-epimer pair shares the same exact mass and fragmentation pattern. In addition, a proper chromatographic separation between both compounds may be complex. Therefore, well-optimized LC gradients are required to avoid misquantification when EA epimers co-occur in food samples. Although several studies have reported LC-MS methods for unambiguous determination of EAs15,16,17,18, the chromatographic method must be studied extensively to achieve adequate separation of the chromatographic peaks to unequivocally identify EAs. However, this is not usually feasible for multi-class methods in which contaminants belonging to different chemical families are simultaneously determined. In this context, a recent study conducted by Carbonell-Rozas, Hernández-Mesa, et al.19 reported an LC-IMS-MS method for the quantification of EAs in wheat and barley samples, using two different TWIMS instruments that provided reproducible CCS values and low limits of quantification (LOQs) to detect any non-compliance in accordance with current legislation. Therefore, the goal of this protocol is to show how the integration of IMS in LC-MS workflows contributes to the separation of isomeric EAs, enhancing the selectivity of the analytical method. Additionally, it illustrates how the generation of CCS libraries through the characterization of analytical standards provides higher confidence for mycotoxin identification. This protocol is designed to clearly explain the benefits of implementing IMS in food safety analysis, taking as an example the determination of EAs in cereals. This protocol addresses the sample treatment based on a QuEChERS procedure, sample analysis by LC-TIMS-MS, and IMS data extraction and interpretation.
Protocol
1. Preparation of stock, intermediate, and working standard solutions
NOTE: Use nitrile gloves, laboratory coat, and safety glasses.
- Prepare individual stock solutions of the 12 EAs (see Table of Materials) at 10,000 ng/mL in 4 mL amber glass vials using acetonitrile. The R-forms (-ine) were previously aliquoted in 25,000 ng portions, whereas the S-forms (-inine) were distributed in 10,000 ng portions. This study began with these aliquots, reaching concentrations of 10,000 ng/mL by diluting the R- and S-forms in 2.5 and 1 mL of acetonitrile, respectively, followed by vortexing for 2 min.
- Prepare an intermediate stock solution at 1,000 ng/mL of total EAs (83.33 ng/mL of each) in a 12 mL amber glass vial by adding 65 µL of each individual alkaloid solution and 7.02 mL of acetonitrile.
NOTE: Once the intermediate stock solution is prepared, individual stock solutions must be dried and stored at -20 °C covered by aluminum foil to avoid epimerization. - Prepare working standard solutions from the intermediate stock solution by pipetting 2.7, 1.8, and 0.9 mL in 4 mL amber glass tubes. Take them to dryness under gentle nitrogen stream and resuspend in 600 µL of acetonitrile.
NOTE: The resulting concentrations were established to reach 1.5, 1, and 0.5x the mL (150 ng/g) when adding 50 µL to 1 g of the sample. These are selected as concentration levels for validation studies (i.e., recoveries, repeatability, etc.). - Divide the 600 µL of each working standard solution in three 2 mL amber glass vials by pipetting 200 µL and take them to dryness.
NOTE: Steps 1.2 to 1.4 are schematically explained in Figure 1. By dividing and drying the working standard solutions, it is possible to fortify in triplicate on three different days, as required by the validation study, without losing the chemical integrity of the EAs. - On each validation day, resuspend one 2 mL amber glass vial of each fortification level in 200 µL of acetonitrile.
NOTE: Each fortification level will be assessed in triplicate using 50 µL per sample; therefore, a total of 150 µL of the solution will be used.
2. Preparation of reagents and solutions
NOTE: Use nitrile gloves, laboratory coat, and safety glasses.
- Prepare a 5 mM ammonium carbonate solution by weighing 24.02 mg of ammonium carbonate and dissolving it in 50 mL of water. Weigh 24.02 mg of ammonium carbonate into a beaker and add approximately 5 mL of water. Dissolve the ammonium carbonate by gently agitating the solution manually, then transfer it to a 50 mL volumetric flask. To reach the final volume of 50 mL, rinse the beaker with additional water and transfer the rising to the volumetric flask.
- Prepare 250 mL of the extraction solution [acetonitrile:5 mM ammonium carbonate (85:15, v/v)] by mixing 212.5 mL of acetonitrile and 37.5 mL of the ammonium solution prepared in step 2.1.
- Prepare 100 mL of a methanol:water mixture (1:1, v/v) to resuspend the sample extracts (step 8.8.).
- For each sample, weigh 150 mg of a dispersive solid phase material C18:zirconia-based sorbent mixture (1:1, w/w) into 15 mL centrifuge tubes for dispersive clean-up stage (step 8.5.).
- Prepare the solvents for chromatographic separation in two different bottles, namely, 1 L of ultrapure water (solvent A) and methanol (solvent B), both containing 0.3% (v/v) formic acid. To achieve this concentration, 3.03 mL of formic acid (99% purity) was added to the bottles.
NOTE: Solvents for the mobile phase should be prepared just before running the LC-IMS-MS analyses.
3. Setting instrumental parameters
NOTE: The instrument used to perform this LC-IMS-MS study was a UHPLC coupled with an IM-HRMS, equipped with a vacuum-insulated probe heated electrospray ionization (VIP-HESI) source. The instrument was operated in positive mode.
- Create a chromatographic method using the acquisition software for the separation of the analytes by setting the following elution gradient: 0 min, 10% B; 2 min, 10% B; 4.5 min, 40% B; 9 min, 45% B; 11 min, 95% B; 12 min, 95% B; 13 min, 10% B; 16 min, 10% B.
- Set the mobile phase flow rate at 0.4 mL/min, the column temperature at 35 °C, and the injection volume at 5 µL. If possible, keep a fixed temperature of 10 °C inside the autosampler.
- Set the IMS parameters as follows: mobility values (1/K0), from 0.1 to 1.5 V·s/cm2; ramp time, 100 ms; accuracy time, 10 ms; duty cycle, 10%; and ramp rate, 9.05 Hz.
- Set the ion source parameters for MS detection as follows: capillary voltage, 2,500 V; nebulizer pressure, 2.5 bar; dry gas flow rate, 8 L/min; dry gas temperature, 200 °C; sheath gas temperature, 450 °C; sheath gas flow rate, 4 L/min. For MS acquisition, select the following parameters, taking into account two scan events: full scan of the entire mass spectrum with a m/z from 20 to 1,300 followed by bbCID (broadband collision-induced dissociation), which generates product ions from any precursor at three fixed collision energy of 24, 36, and 50 eV. Perform ionization and data acquisition in positive polarity mode.
- Save this acquisition method file for further use.
4. Data acquisition from EAs analytical standards
NOTE: Use nitrile gloves, laboratory coat, and safety glasses for step 4.1 only.
- Prepare two 1.5 mL amber vials with 500 µL of intermediate solution at 1,000 ng/mL and another vial with a mixture of solvents A and B (1:1, v/v) as washing solution.
- Calibrate the timsTOF instrument as indicated by the manufacturer and select positive polarity for both IMS and MS acquisitions.
- Place the C18 column (see Table of Materials) inside the LC oven, with the temperature set to 35 °C as indicated in the acquisition method file saved in step 3.5.
NOTE: Make sure both ends are well tightened; otherwise, mobile phase leakage will be observed. - Condition the LC column to allow a mobile phase flow rate of 0.1 mL/min and select a solvent A and B mixture ratio 90:10 (%) as the initial mobile phase composition ratio.
- Wait until the column pressure is stable (~130 bar).
- Increase the mobile phase flow rate to 0.2 mL/min, wait until the pressure stabilizes, and increase it again to 0.3 mL/min.
- Once the pressure stabilizes again, set the flow rate to 0.4 mL, which will be the working flow rate as detailed in the acquisition method file saved in step 3.5, and wait until the pressure stabilizes around 350 bar.
NOTE: It is important not to directly increase the flow from 0.2 to 0.4 mL/min. A strong change in the flow provokes high-pressure peaks that may harm the chromatography column. - While the column is being conditioned, write the worklist, including two samples corresponding to the two vials containing the EAs intermediate solution at 1,000 ng/mL, as indicated in step 4.1, and the wash vial. Analyze the wash vial twice, at the beginning and end of the sample batch.
- In Method file column of the worklist, load the acquisition method file previously saved in step 3.5.
- Create a folder to save all acquired data files and route all samples by selecting the folder in the Sample path column.
NOTE: Be sure to use the same folder to save all the files generated during the validation study for further processing. During the column conditioning, the values at which pressure stabilizes may vary slightly. Nonetheless, ensure that the pressure is stable before increasing the flow rate again to preserve column integrity. Moreover, when the working flow rate is reached, pressure must also be stable to achieve reproducible chromatographic separation. - Go to the upper toolbar of the software and run the worklist.
5. Data treatment for the creation of a quantification method
- Once the worklist is executed, open the qualitative software.
- Load all analyzed samples by clicking File | Open worklist.
- Right-click on a sample corresponding to the EAs intermediate solution at 1,000 ng/mL, go to Edit chromatogram, then select Type | Extracted ion chromatogram, and enter the theoretical molecular mass of the protonated ion related to each EA. Then, click Add, and finally, OK. Since the set of analytes is formed by six EAs and their related epimers that share the same exact mass, six extracted ion chromatograms will appear.
NOTE: The Width parameter changes how narrow the m/z window is. It is recommended to select a sufficiently wide m/z window in case of difficulties in finding the chromatographic peak of the analyte of interest. However, it is advisable to set narrow m/z windows to avoid possible interferences (below 0.05 units). - In each extracted ion chromatogram, two chromatographic peaks related to the main EA and its epimer will appear. Right-click while selecting the whole area of one peak. Consequently, the ion mobility spectrum will pop up right below.
- Go to the x-axis of the ion mobility spectrum, right-click, and then click on Collision Cross Section. Write down the CCS value.
NOTE: If the CCS values do not automatically appear, go to Calibrate in the upper toolbar, then Mobility calculator, and manually type the mobility value to obtain their corresponding CCS. - Select the area corresponding to the other chromatographic peak within the same ion chromatogram and repeat steps 5.4. and 5.5.
- Similarly, the fragmentation pattern will also appear after step 5.4. Based on the fragmentation pattern reported in literature and databases, compare and choose the most intense product ion as a complementary identification point.
- Write down the retention time, CCS value, and exact mass of the main ion adduct (usually the protonated ion) of each EA to create a quantitation method.
- Repeat steps 5.4 to 5.8. for the remaining analytes.
- Select all the molecular masses generated in step 5.3 by clicking on them while pressing Ctrl. Then, right-click and select Copy. Go to the other sample corresponding to the EAs intermediate solution, right-click on it and select Paste. The ion chromatograms and mobility spectra will be automatically extracted.
- Double-check the identification parameters for all the analytes in this second sample.
6. Creation of a data processing method for the routine determination of ergot alkaloids
- Open the software and click on the Method Management tab.
- Click on Analyte settings and enter the name of each EA alongside its retention time, CCS value, and the mass of the protonated adducts collected in steps 5.4. to 5.9. Change the tolerance of each identification point as necessary, although the default settings are recommended for the application of this protocol.
NOTE: The higher the tolerance values set, the higher the possibility of dealing with false positives, since a greater deviation from the theoretical values is accepted. Similarly, tolerance values that are too stringent may lead to false negatives. This happens when the deviation from the theoretical value is solely caused by the instrument's performance. - Save the quantitation method for further use.
7. Sampling
- Acquire samples of wheat and oat in supermarkets, preferably in 1 kg containers, following the guidelines for sampling established by the European Commission20. If 1 kg packages are not available, just make sure to have, at least, 50 g of samples to complete the whole analysis.
- Mill the samples using a grinder. In this study, a blade grinder was used to mill the entire content of the packages, which were then returned to their original packages for homogenization.
NOTE: Clean the grinder thoroughly between samples to avoid cross-contamination. Use a laboratory coat. - Aliquot the homogenized samples in 50 mL centrifuge tubes and store them in cool and dry conditions.
8. Sample preparation
- Prepare a schedule to perform the validation study for three non-consecutive days (to assess in-house reproducibility). For each day, assay three concentration levels by fortifying samples at 225 ng/g (1.5 mL), 150 ng/g (ML), and 75 ng/g (0.5 mL). Study each level in triplicate, representing nine spiked samples plus another three blank samples per day.
NOTE: Considering the 3 validation days, a total of 36 samples will be required alongside another 8 blank samples intended for preparing the matrix-matched calibration curve. Use nitrile gloves, laboratory coat, and safety glasses for this entire section of the protocol. - Weigh 1 g of sample into a 50 mL centrifuge tube.
NOTE: When performing the validation study, fortify the samples just after weighing with 50 µL of the corresponding working standard solution detailed in steps 1.3. and 1.5. - Add 4 mL of the extraction solution [acetonitrile:5 mM ammonium carbonate aqueous solution (85:15, v/v)]. Vortex the sample for 1 min.
- Centrifuge the sample for 5 min at 9,750 × g and 4 °C.
NOTE: The capacity of the centrifuge may be a limiting factor in the procedure, so it is recommended to simultaneously extract only the exact number of samples that will fit in the centrifuge. - Transfer the whole supernatant to the 15 mL centrifuge tube containing 150 mg of the clean-up sorbent (1:1, w/w) mixture. Vortex the sample for 1 min.
- Centrifuge the tube for 5 min at 9,750 × g and 4 °C.
- Collect the supernatant and place it in a 4 mL amber glass vial. Evaporate the extract under a gentle stream of nitrogen.
NOTE: Samples must be kept dried at -20 °C if not analyzed on the same day. - Reconstitute the sample in 750 µL of the methanol:water (1:1, v/v) mixture.
- Transfer the extract to a 2 mL syringe and filter it through a 0.22 µm nylon filter into a 1.5 mL amber chromatographic vial.
- Collect the reconstituted extracts of the eight blank samples intended for the matrix-matched calibration curve into a 12 mL amber glass vial (each sample is resuspended in 750 µL, representing a total volume of 6 mL).
- Pipette 1 mL of the intermediate solution at 1,000 ng/mL (83.33 ng/mL for each ergot alkaloid) (step 1.2) into a 2 mL amber glass vial, dry the solvent under a gentle stream of nitrogen, and resuspend in 1 mL of the previously collected blank sample extract.
NOTE: This mix at a concentration of 1,000 ng/mL (83.33 ng/mL for each EA) will be used to prepare the matrix-matched calibration curve. - Prepare ten 2 mL amber glass vials and place 450 µL of blank sample extract (step 8.10) in each one and mark them with numbers from 1 to 10. Each vial will be a point of the calibration curve.
NOTE: In this protocol, 450 µL was used since it was the lowest value that the LC device could reach inside the vial without using inserts. If the needle does not reach that far into the vial, increase the volume and, consequently, calculate how many extra blank samples should be extracted considering that each one is resuspended in 750 µL of methanol:water (1/1, v:v) mixture. - Build the calibration curve by serial dilution. Transfer 450 µL of the mix prepared in step 8.11. into vial 1, which already contains 450 µL of blank sample extract, to achieve a concentration of 41.67 ng/mL (the highest calibration point). Vortex the vial for 2 min.
NOTE: The volume of the mix must match the volume of blank sample extract to dilute twice in each step. - Transfer 450 µL from vial 1 into vial 2, which already contains 450 µL of blank sample extract, to reach a concentration of 20.83 ng/mL (the second highest calibration point).
- Repeat step 8.14 (i.e., from vial 2 to vial 3, and so on) and continue subsequently until the ten calibration points are obtained. The concentration range for each EA will then be: 41.67, 20.83, 10.42, 5.21, 2.60, 1.30, 0.65, 0.33, 0.16, and 0.08 ng/mL.
- Prepare a standard mixture at 1,000 ng/mL (83.33 ng/mL for each EA) as detailed in step 8.11 but using 1 mL of methanol:water (1:1, v/v) mixture.
- Prepare a standard calibration curve as detailed in steps 8.13 to 8.15 but using 450 µL of methanol:water (1:1, v/v) mixture for the serial dilutions. This calibration curve will be used to evaluate the matrix effect by comparing it with the matrix-matched calibration curve.
9. Quantitative data treatment
- Initialize and run the instrument following the instructions described in steps 4.1. to 4.11. See Figure 2 for the worklist used in this protocol.
NOTE: When writing the worklist for calibration curves, inject them before the fortified samples starting with the standard calibration curve from the lowest to the highest concentration, a wash vial containing methanol:water (1:1, v/v), and the matrix-matched calibration curve from the lowest to the highest concentration. - Open the quantitative software and navigate to TASQ Quick tab | Import batch at the bottom left.
- Select the folder created in step 4.10. containing all raw data (files related to the information acquired after the LC-IMS-MS analysis for all samples included in the worklist).
- Once imported into the software, go to Process batch, right below Import batch.
- Choose the samples to process (i.e., the 27 spiked samples, 9 blanks, and both calibration curves) and the data processing method previously created in steps 6.1. to 6.3. A window will pop up with different parameters to set (i.e., the type of integrator to assess the chromatographic peaks or the type of analytical signal to consider, among others). Change those parameters as desired, although the default values are suitable for the execution of this protocol.
- Go to Review screening after data processing is complete and check if there are any issues related to the automatic integration, such as the selection of incorrect chromatographic peaks that may be too close to each other. If so, narrow the values referring to retention time tolerance, CCS values tolerance, and/or mass error initially established in the quantitative method file and reprocess the data. If automatic peak integration still selects incorrect peaks, manually integrate the peak by selecting the area in the chromatogram that appears in Review Screening window (to do this, take into account the analytical parameters obtained for the EA standards in section 5).
NOTE: It is very important to review each sample, as some main EA-epimer pairs have very similar retention times and may be misidentified by the software. - When all peaks have been successfully integrated for all samples, click on Batch management tab in the upper left corner and indicate the type of sample associated with each analysis: solvent, blank, quality control, sample, or calibration point. In the latter case, assign each calibration point at a level with a number.
- In the same Batch management tab, navigate to the bottom window called Batch concentration and specify a concentration value for each level established in the previous step. Once this is done, click on Save concentrations.
- Click on Quantify batch and change the parameters as desired (i.e., the model to fit the calibration data, weighting, whether the model is forced to cross zero, etc.). The default values are suitable for the execution of this protocol except for the weighting of the calibration curves, which was switched to 1/x.
NOTE: Since the software can only consider one calibration curve at a time, it is necessary to repeat step 9.9 twice to have information about standard and matrix-matched calibration curves. In this protocol, matrix-matched calibration curves were used for the quantification of analytes, so the solvent calibration curves were assessed first, calibration data were extracted as later explained in step 9.13, and, finally, the matrix-matched calibration curves were selected to perform the quantification of samples. - Once the quantification of ergot alkaloids has been performed according to the quantification method file, click on the Quantitation tab in the upper part of the screen.
- If the default parameters set in step 9.9 do not provide good linearity and precision, adjust the calibration curves by changing the fitting and the weight by right-clicking on the graph with the aim of having a good linear model (R2 > 0.99) with adequate precision on each calibration level (deviation below 20%).
NOTE: The software will mark in red the cells from those calibration levels that do not provide proper precision. - Once finished, right-click on the batch folder at the far left of the screen and select Generate report. Then, select Analyte quantitation to export the report to the desired format.
Results
First, working standard solutions were injected into the LC-IMS-MS instrument to obtain all the identification features (i.e., retention time, CCS, and mass spectra) of each EA analyzed here. Since the identification parameters, except the exact mass, were initially unknown, the acquisition method was based on a two-scan event, starting with a full scan of the entire mass spectrum followed by a bbCID. The retrospective way of approaching this study is enabled by the Q-TOF high-resolution mass spectrometer, which acquires...
Discussion
The successful use of this protocol is based on the optimization of the extraction procedure, previously carried out by Carbonell-Rozas et al.17, who implemented the use of an extraction solvent effective enough to extract EAs from complex food matrices such as barley and wheat, and a clean-up that provided relatively low SSE values. The choice of extraction solvent represents a critical step considering the chemical characteristics of the analytes and the lability of EAs to decomposition and epim...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This research was funded by the Consejería de Universidad, Investigación e Innovación - Junta de Andalucía (PROYEXCEL_00195) and the postdoctoral grant given by the Generalitat Valenciana and European Social Fund+ (CIAPOS/2022/049). The authors thank the "Centro de Instrumentación Científica (CIC)" at the University of Granada for providing access to the analytical instrumentation used in this protocol.
Materials
| Name | Company | Catalog Number | Comments |
| Acetonitrile | VWR | 83640.32 | |
| Amber glass tubes 4 mL | VWR | 548-0052 | |
| Amber glass tubes 12 mL | VWR | 548-0903 | |
| Amber vials 1.5 mL | Agilent | 5190-9063 | |
| Ammonium carbonate | Fluka | 9716 | |
| Analytical balance BAS 31 | Boeco | 4400519 | |
| Balance CP 323 S | Sartorius | 23-84182 | |
| C18 | Supelco | 52604-U | |
| Centrifuge tubes, 15 mL | VWR | 525-1082 | |
| Centrifuge tubes, 50 mL | VWR | 525-0155 | |
| Centrifuge Universal 320 R | Hettich | 1406 | |
| Compass HyStar | Bruker | Acquisition software | |
| DataAnalysis | Bruker | Qualitative software | |
| Elute PLUS UHPLC | Bruker | ||
| EVA EC-S evaporator | VLM | V830.012.12 | |
| Formic acid GR for analysis ACS, Reag. Ph Eur | Merck | 100264 | |
| Grinder TitanMill300 | Cecotec | 1559 | |
| Methanol | VWR | 83638.32 | |
| Milli-Q water purification system (18.2 MΩ cm) | Millipore | ZD5211584 | |
| Pipette tips 1- 5 mL | Labortecnic | 162005 | |
| Pipette tips 100 - 1000 µL | Labortecnic | 1622222 | |
| Pipette tips 5 - 200 µL | Labortecnic | 162001 | |
| Pippette Transferpette S variable, DE-M 10 - 100 µL | BRAND | 704774 | |
| Pippette Transferpette S variable, DE-M 100 - 1000 µL | BRAND | 704780 | |
| Pippette Transferpette S variable, DE-M 500 - 5000 µL | BRAND | 704782 | |
| Syringe 2 mL | VWR | 613-2003 | |
| Syringe Filter 13 mm, 0.22µm | Phenomenex | AF-8-7707-12 | |
| TASQ | Bruker | Quantitative software | |
| timsTOFPro2 IM-HRMS | Bruker | ||
| Vortex Genie 2 | Scientific Industries | 15547335 | |
| Zorbax Eclipse Plus RRHD C18 column (50 x 2.1 mm, 1.8 µm particle size) | Agilent | 959757-902 | |
| Z-Sep+ | Supelco | 55299-U | Zirconia-based sorbent |
| Ergot alkaloids | CAS registry sorbent | ||
| Ergocornine (Eco) | Techno Spec | E178 | 564-36-3 |
| Ergocorninine (Econ) | Techno Spec | E130 | 564-37-4 |
| Ergocristine (Ecr) | Techno Spec | E180 | 511-08-0 |
| Ergocristinine (Ecrn) | Techno Spec | E188 | 511-07-9 |
| Ergokryptine (Ekr) | Techno Spec | E198 | 511-09-1 |
| Ergopkryptinine (Ekrn) | Techno Spec | E190 | 511-10-4 |
| Ergometrine (Em) | Romer Labs | "002067" | 60-79-7 |
| Ergometrinine (Emn) | Romer Labs | LMY-090-5ML | 479-00-5 |
| Ergosine (Es) | Techno Spec | E184 | 561-94-4 |
| Ergosinine (Esn) | Techno Spec | E194 | 596-88-3 |
| Ergotamine (Et) | Romer Labs | "002069" | 113-15-5 |
| Ergotaminine (Etn) | Romer Labs | "002075" | 639-81-6 |
References
- Kanu, A. B., Dwivedi, P., Tam, M., Matz, L., Hill, H. H. Ion mobility-mass spectrometry. J Mass Spectrom. 43 (1), 1-22 (2008).
- Gabelica, V., et al. Recommendations for reporting ion mobility Mass spectrometry measurements. Mass Spectrom Rev. 38 (3), 291-320 (2019).
- Feuerstein, M. L., et al. Comparability of steroid collision cross sections using three different IM-HRMS technologies: An interplatform study. J Am Soc Mass Spectrom. 33 (10), 1951-1959 (2022).
- Regueiro, J., Negreira, N., Berntssen, M. H. G. Ion-mobility-derived collision cross section as an additional identification point for multiresidue screening of pesticides in fish feed. Anal Chem. 88 (22), 11169-11177 (2016).
- Regueiro, J., Negreira, N., Hannisdal, R., Berntssen, M. H. G. Targeted approach for qualitative screening of pesticides in salmon feed by liquid chromatography coupled to traveling-wave ion mobility/quadrupole time-of-flight mass spectrometry. Food Control. 78, 116-125 (2017).
- Hernández-Mesa, M., Monteau, F., Le Bizec, B., Dervilly-Pinel, G. Potential of ion mobility-mass spectrometry for both targeted and non-targeted analysis of phase II steroid metabolites in urine. Anal Chim Acta: X. 1, 100006 (2019).
- George, A. C., et al. Interplatform comparison between three ion mobility techniques for human plasma lipid collision cross sections. Anal Chim Acta. 1304, 342535 (2024).
- Mairinger, T., Causon, T. J., Hann, S. The potential of ion mobility-mass spectrometry for non-targeted metabolomics. Curr Opin Chem Biol. 42, 9-15 (2018).
- Hernández-Mesa, M., Escourrou, A., Monteau, F., Le Bizec, B., Dervilly-Pinel, G. Current applications and perspectives of ion mobility spectrometry to answer chemical food safety issues. TrAC Trend Anal Chem. 94, 39-53 (2017).
- European, C. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off J Eur Union. 119, 103-157 (2023).
- Carbonell-Rozas, L., et al. Occurrence of egot alkaloids in major and minor cereals from Northern Italy: A three harvesting years scenario. J Agricl Food Chem. 71 (42), 15821-15828 (2023).
- Arroyo-Manzanares, N., Gámiz-Gracia, L., García-Campaña, A. M., Diana Di Mavungu, J., De Saeger, S., Jean-Michel Mérillon, J. -. M., Ramawat, K. G. . Fungal Metabolites. , (2016).
- van Dongen, P., de Groot, A. History of ergot alkaloids from ergotism to ergometrine. Eur J Obstet Gynecol Reprod Biol. 60 (2), 109-116 (1995).
- European Food Safety Authority. Human and animal dietary exposure to ergot alkaloids. EFSA J. 15 (7), e04902 (2017).
- Nam, M., Kim, D., Kim, M. -. S. Simultaneous determination of total ergot alkaloids in wheat flour by Orbitrap mass spectrometry. Food Chem. 441, 138363 (2024).
- García-Juan, A., León, N., Armenta, S., Pardo, O. Development and validation of an analytical method for the simultaneous determination of 12 ergot, 2 tropane, and 28 pyrrolizidine alkaloids in cereal-based food by LC-MS/MS. Food Res Int. 174 (Pt 1), 113614 (2023).
- Carbonell-Rozas, L., Mahdjoubi, C. K., Arroyo-Manzanares, N., García-Campaña, A. M., Gámiz-Gracia, L. Occurrence of ergot alkaloids in barley and wheat from Algeria. Toxins. 13 (5), 316 (2021).
- Carbonell-Rozas, L., Gámiz-Gracia, L., Lara, F. J., García-Campaña, A. M. Determination of the main ergot alkaloids and their epimers in oat-based functional foods by ultra-high performance liquid chromatography tandem mass spectrometry. Molecules. 26 (12), 3717 (2021).
- Carbonell-Rozas, L., et al. Ion mobility-mass spectrometry to extend analytical performance in the determination of ergot alkaloids in cereal samples. J Chromatogr A. 1682, 463502 (2022).
- European Commission. Commission Implementing Regulation (EU) 2023/2782 of 14 December 2023 laying down the methods of sampling and analysis for the control of the levels of mycotoxins in food and repealing Regulation (EC) No 401/2006. Off J Eur Union. , (2023).
- Carbonell-Rozas, L., Vander Cruyssen, L., Dall'Asta, C., Leggieri, M. C., Battilani, P. Fit-for-purpose method development to determine co-occurring multiclass mycotoxins in apple and apple puree samples. Food Anal Methods. 16 (8), 1403-1412 (2023).
- Laouni, C., et al. Emerging mycotoxin occurrence in chicken feed and eggs from Algeria. Mycotoxin Res. 40, 447-456 (2024).
- Ben Hassouna, K., et al. Mycotoxin occurrence in milk and durum wheat samples from Tunisia using dispersive liquid-liquid microextraction and liquid chromatography with fluorescence detection. Toxins. 15 (11), 633 (2023).
- Narváez, A., et al. Occurrence and exposure assessment of mycotoxins in ready-to-eat tree nut products through ultra-high performance liquid chromatography coupled with high resolution Q-orbitrap mass spectrometry. Metabolites. 10 (9), 344 (2020).
- Arroyo-Manzanares, N., Rodríguez-Estévez, V., García-Campaña, A. M., Castellón-Rendón, E., Gámiz-Gracia, L. Determination of principal ergot alkaloids in swine feeding. J Sci Food Agric. 101 (12), 5214-5224 (2021).
- Pereira, V. L., Fernandes, J. O., Cunha, S. C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC-MS. Food Chem. 182, 143-149 (2015).
- Tuzimski, T., Szubartowski, S. Method development for selected bisphenols analysis in sweetened condensed milk from a can and breast milk samples by HPLC-DAD and HPLC-QqQ-MS: Comparison of sorbents (Z-SEP, Z-SEP Plus, PSA, C18, chitin and EMR-lipid) for clean-up of QuEChERS extract. Molecules. 24 (11), 2093 (2019).
- Łozowicka, B., Rutkowska, E., Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ Sci Pollut Res. 24 (8), 7124-7138 (2017).
- Schummer, C., Xandonella, I., van Nieuwenhuyse, A., Moris, G. Epimerization of ergot alkaloids in feed. Heliyon. 6 (6), e04336 (2020).
- Cherewyk, J. E., Grusie-Ogilvie, T. J., Parker, S. E., Blakley, B. R., Al-Dissi, A. M. The impact of storage temperature and time on ergot alkaloid concentrations. Toxins. 15 (8), 497 (2023).
- Silva, &. #. 1. 9. 4. ;., Mateus, A. R., Barros, S. C., Silva, A. S. Ergot alkaloids on cereals and seeds: Analytical methods, occurrence, and future perspectives. Molecules. 28 (20), 7233 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved