A subscription to JoVE is required to view this content. Sign in or start your free trial.
A Rapid, Simple Workflow for Quantification of External Adult Drosophila Structures
In This Article
Summary
Here, we present a rapid, low-cost, workflow for high-resolution imaging of adult Drosophila eyes to quantify patterning and growth defects. We describe our protocol for sample preparation by point-mounting, high-resolution image acquisition, and image analysis.
Abstract
The Drosophila compound eye is a precisely patterned tissue that has revealed molecular mechanisms and biological processes that drive morphogenesis. It is a simple structure of repeating unit eyes, termed ommatidia, that is used to characterize genetic interactions and gene functions. Mutations that affect eye architecture can be easily detected and analyzed; hence, this system is frequently used in under-resourced institutions. Further phenotypic analysis often includes a Scanning Electron Microscope (SEM) to generate high-magnification images suitable for quantitative analysis. However, SEMs are expensive and require costly reagents; sample preparation spans days; and, often, they need full-time staff for sample preparation and instrument maintenance. This limits their utility at under-resourced institutions or during budgetary austerity. In entomology, the use of high-resolution digital imaging technology is a common practice for the identification and characterization of species. This paper describes a method that combines strategies and allows for high-resolution digital imaging of adult Drosophila structures and quantitative analysis using the open software ImageJ. The workflow is a rapid and student-friendly alternative that remedies the limitations of underfunded and under-resourced research facilities with a cost-effective and rapid approach to quantitative phenotypic analysis.
Introduction
Drosophila melanogaster is a powerful genetic model organism that has been used for decades to elucidate molecular signaling pathways and cellular behaviors. Many of the evolutionarily conserved signaling pathways that are essential for multicellular development were first identified and their mechanism of action defined in Drosophila. About 65-75% of all human disease-associated genes have orthologs in Drosophila1,2. The adult Drosophila eye is an important model that has allowed for unbiased genetic screens that facilitated the discovery of important conserved genes implicated in human diseases, including cancer3,4, neurodegeneration5, and metabolic disorders6.
The Drosophila eye is composed of ~800 unit eyes, termed ommatidia, that are precisely arrayed in a hexagonal pattern across the surface of the adult eye7. Each ommatidium is composed of eight photoreceptor neurons that occupy a distinct location within an asymmetrical trapezoid. These are supported by four non-neural cone cells and two primary pigment cells that secrete lens and pseudo-cone to focus light onto the light-sensing rhabdomeres of the photoreceptor neurons. Neighboring ommatidia are separated by a single row of interommatidial lattice cells, comprised of secondary pigment cells, tertiary pigment cells, and mechanosensory bristle complexes8,9,10.
Perturbations in eye development are visible in adult eyes as increased or decreased eye size, abnormal abundance or structure of lenses or bristles, or as a "rough eye" where the normally invariant hexagonal patterning is disrupted such that a row of ommatidia can no longer be followed across the surface of the eye. These phenotypes can be scored at the gross tissue level using dissecting microscopes. Detailed analysis of phenotypes traditionally includes scanning electron microscopy followed by quantitative image analysis11. However, scanning electron microscopy requires expensive instrumentation, costly reagents, sample preparation that spans days, and often, full-time staff to maintain.
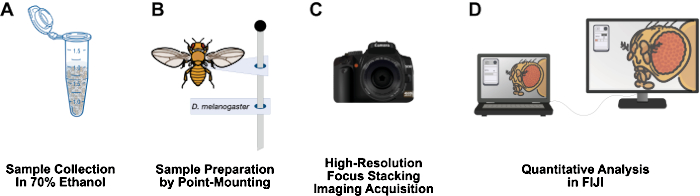
Figure 1: Workflow for imaging adult Drosophila structures. (A) Collect and fix adult Drosophila in 70% ethanol. (B) Prepare samples for imaging by point mounting and affixing to pins. (C) Acquire high-resolution images through focus stacking and integration. (D) Quantify images using FIJI. Please click here to view a larger version of this figure.
This paper presents a workflow that is relatively inexpensive, has a short sample preparation time, can easily be set up on a 3-foot lab bench, does not require hazardous materials, and could be a long-lived addition to Drosophila research labs (Figure 1). Point mounting is an entomological technique used to air dry and preserve small, soft-bodied insects, such as Drosophila12. This method relies on combining microscope objectives with high-resolution DSLR cameras for effective magnifications of 10x to 1,000x. The limited depth of field inherent to macrophotography is overcome by focus stacking: stitching together a series of images with the focal plane moving through the specimen of interest13. This method yields high-resolution images suitable for the quantification of phenotypes and could easily be adapted for other structures of interest, such as the wing, leg, thorax, and abdomen. The image analysis workflow uses the free image analysis program FIJI (NIH ImageJ). This methodology makes sample preparation, high-resolution imaging, and analysis accessible for undergraduate students and scientists at under-resourced institutions.
Protocol
1. Adult Drosophila collection and fixation
- Set up Drosophila crosses or select strains and place them in vials containing fly food. Incubate the vials at the desired temperature (usually 25 °C) until the flies have developed and adults eclose (~10-14 days at 25 °C).
- Anesthetize the flies with CO2 and place them on a CO2 pad.
- Sort the flies with a feather and select the individuals with the desired phenotype (e.g., straight wings). Make a feather fly sorter by trimming a goose feather to fit in the tapered end of a 1 mL serological pipette.
- Prepare a 1.7 mL microcentrifuge tube with 1 mL of 70% ethanol. Place the selected flies in the microcentrifuge tube and put on ice. Store the microcentrifuge tubes at 4 °C overnight (Figure 2A).
NOTE: Do not preserve flies in ethanol for more than 24 h. Long-term storage of flies in 70% ethanol will result in loss of eye and body pigment.
2. Sample preparation by point-mounting
NOTE: Drosophila are soft-bodied insects that become brittle and collapse when air-dried; therefore, this protocol requires samples to be imaged the same day as they are mounted. Work in small sets of ~5 flies at a time to prevent sample loss. Increase the number of samples in a set based on efficiency. Specimens that require more time before imaging can be dehydrated through an increasing concentration series of hexamethyldisilazane (HMDS)14.
- Cut small triangular points (7.1 mm x 2.7 mm) from archival 65 lb cardstock using a specialized point punch. Prepare points by bending the tip (narrowest 25%) to a 90° angle with Dumont #5 fine-tip forceps (Figure 2B).
- Using Dumont #5 fine-tip forceps, remove the flies from the microcentrifuge tubes (step 1.4). Gently blot the flies with lint-free lab tissue to remove excess ethanol. Position each fly on its left side on an index card under a dissecting microscope.
NOTE: Remove the flies from the tube by holding onto an anatomical structure that is not the area of interest-when imaging the head, we hold samples by the wing or leg. Do not hold samples by the abdomen, as that structure is used to glue the fly onto the card point. - Prepare the hide glue, adjusting its consistency to the desired viscosity. Mix 1-2 drops of hide glue with 1-2 drops of deionized (DI) water, and mix with a transfer pipet on an index card. Pick up a prepared card point at the broad end with forceps, and place a small amount of the diluted glue on the bent tip of the point by dabbing it into the glue-water mix (Figure 2C).
NOTE: The glue should be spreadable but not runny. - Apply the bent tip of the point to the anterior side of the right abdomen around abdominal segments 2-3 (Figure 2C). Before the glue dries, make slight adjustments to the fly such that the anterior-posterior axis of the fly is perpendicular to the bent tip of the point.
- Insert a No. 3 mounting pin into the wide end of the card-point (Figure 2D) and secure it to an insect pinning block (Figure 2E). Label each pin or row of pins with the corresponding genotype.
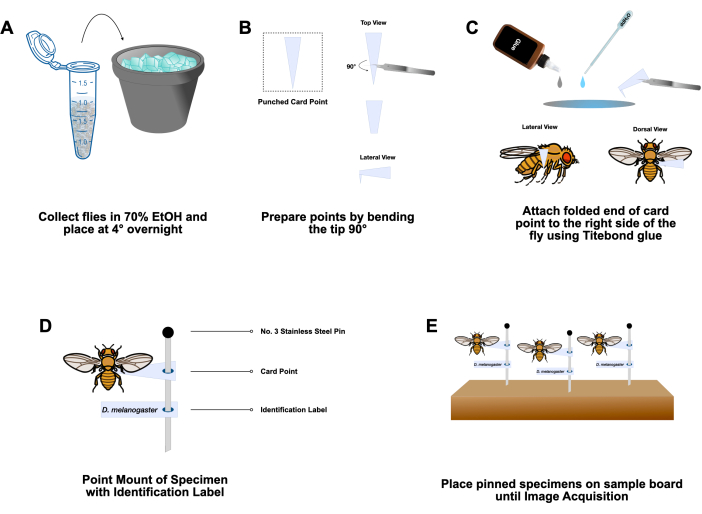
Figure 2: Sample preparation. (A) Adult Drosophila are sorted based on phenotypic markers and collected into labeled microcentrifuge tubes containing 70% ethanol on ice. Flies are stored at 4° C overnight. (B) Paper card points are prepared by bending the narrow end 90° from the rest of the card using a pair of #5 forceps. (C) Flies are recovered from tubes and briefly allowed to air dry. Hide glue is applied to the small, folded end of the prepared card point and glued to the adult fly at abdominal segments 2-3. (D) Specimens are mounted, with an identification label, onto a #3 stainless steel insect pin. (E) Pinned specimens are stored on a sample board until ready for image acquisition. Please click here to view a larger version of this figure.
3. High-resolution focus stacking imaging acquisition
- Acquire high-resolution photographs of fly eyes with an assembled and customized focus stacking imaging system assembled and customized.
- Capture photographs with a DSLR Camera Body with a 70-200 mm telephoto lens connected to a 20x Apo Microscope Objective via a 77 mm lens adapter.
- Ensure that the specimen is illuminated with a flash through a diffuser (Figure 3).
- Control the Z positioning using a Stackshot Controller and Macro Rail.
- Connect the camera, flash, and motorized stage to a heavy-duty anodized aluminum tripod.
- Position each point-mounted sample on a universal stage gimbal with the head oriented so that the eye is facing toward the lens. Make head position adjustments by gently moving the head with the forceps.
CAUTION: Large and fast adjustments can result in accidental decapitation. - With the camera tethered to a laptop computer, adjust the acquisition settings in the software. Photograph specimens at 20x magnification with these settings: Flash Power 1/32, Shutter Speed 1/200, Aperture F2.8, and ISO 400. Ensure that any Autofocus and Image Stabilization features are turned off.
NOTE: These settings balance optimal flash illumination, shutter speed, and depth of field. They would need to be adjusted for other magnifications and/or lens combinations. - Set the location for saving the resulting image stack (10-50 images) to the desired file folder. Ensure enough storage capacity for the images (~8.5 MB per image).
- Adjust the focus stack settings on the Stackshot control unit in Auto Distance mode.
- Set the step size to 5 µm and calculate the number of steps by setting the start and stop positions of the focus stack.
- View the specimen in the LiveView Mode and with the camera in Auto Shoot Mode to identify the start and stop positions.
- Move the rail so that the closest part of the specimen is in focus (set start position), then move to where the furthest feature of interest is in focus (set end position).
- Return the camera to the Manual Shoot Mode and start the image acquisition from the Stackshot control unit.
NOTE: Image acquisition time depends on the size of the specimen. The greater the depth of field necessary for large specimens, the more slices are included in the image stack, which will extend the overall acquisition time. - Open files in the referenced focus stacking software. Generate a stacked image by clicking Stack | Align & Stack All (PMax).
- Save the final image to the computer's hard drive as a .tif file by clicking File | Save Output Image.
NOTE: Depending on the resolution of the stacked image file and the number of specimens imaged, large external hard drives (1 TB) may be necessary for image backup. In this protocol, stacked images are approximately 100 MB each before being compressed.

Figure 3: Image acquisition. (A) Imaging apparatus with parts labeled as follows: a) DSLR Camera Body; b) telephoto lens; c) 20x Apo Microscope Objective and Adapter; d) Flash; e) Lens and Dome Diffusers; f) Stackshot Controller, Macro Rail, and Rotary Stage; g) Universal Stage Gimbal; h) Tripod. (B) Imaging apparatus with light diffuser in place. (C) Close-up of mounted specimen in position for imaging. Please click here to view a larger version of this figure.
4. FIJI analysis workflow to calculate adult eye area
- For image analysis, obtain the FIJI software15 from the ImageJ.net website.
- Choose images for analysis where the eye is centered and aligned with adequate lighting and minimal peripheral blurring, indicating proper alignment with the camera.
- Calibrate the image scale.
- Download the scale bar image for 20x magnification that correlates to 500 µm. Alternately, at the time of image acquisition, photograph a ruler using the same settings. Open the image in FIJI Software.
- Measure the length of scale bar (Figure 4A). Use the Straight Line Tool to exactly trace the line. Click Analyze | Measure. This pixel distance is equivalent to 500 µm (Figure 4B).
- Calculate pixels per micron. Use this to convert pixel measurements into micrometer measurements.
- Open the stacked image file in FIJI (Figure 4C).
- Select Magnifying Glass from the toolbar to enlarge the area of focus. Try to fill the screen with the eye and immediate surrounding head cuticle (Figure 4D).
- Select Freehand Select Tool from the toolbar. Outline the retinal area as closely as possible, following the contours of the outermost row of ommatidia (Figure 4E). To remove part of the selection, hold down the option button and select the pixels to remove. To add to the selection, hold down the option and shift buttons and select the pixels to add.
- To calculate the area, select Analyze | Measure from the top menu (Figure 4F). A new window will appear with area, mean, minimum, and maximum parameters. Copy and paste these data into a spreadsheet for documentation and conversion from pixels to micrometer measurements.
- Perform appropriate statistical analyses.
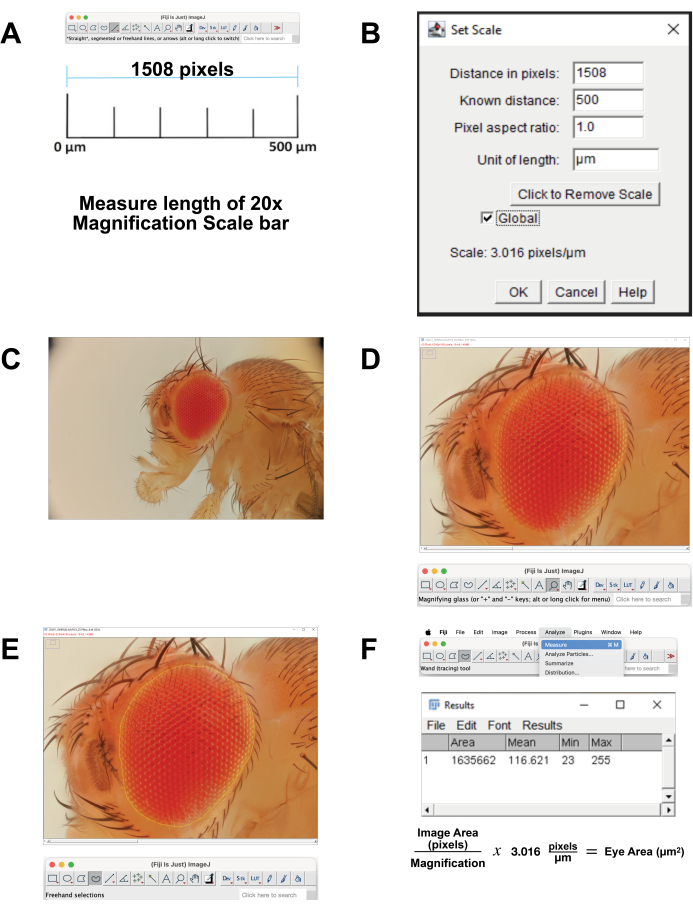
Figure 4: Image analysis in FIJI. (A) Scale the original image. Download the calibration image and measure the length of the 500 µm bar. (B) Adjust the scaling using the Set Scale function. (C) Open the stacked image. (D) Magnify the image so that the eye is centered and nearly full screen. (E) Use the Freehand Select tool to outline the eye at the border between the outermost row of ommatidia and the surrounding cuticle. (F) Measure the area within the selected region is calculated by clicking Analyze | Measure | Area. Please click here to view a larger version of this figure.
Results
The Drosophila eye is an excellent model system for studying tissue patterning, growth control, and cell death. We recently published a study investigating how intracellular pH (pHi) influences tissue growth. First, we established a genetic system where overexpression of the sodium-proton exchanger DNhe2 (the ortholog of mammalian NHE1) in the developing eye causes patterning defects and increased proliferation16. Increased proliferation with higher pHi is observed across species...
Discussion
Here we describe a method for sample preparation, high-resolution imaging, and analysis of adult Drosophila structures. The Drosophila eye is a genetically tractable model system that has yielded critical insights into molecular mechanisms underlying diseases including cancer19, neurodegeneration20 and metabolic diseases21. In particular, cancer patient "avatars" are generated where transgenic Drosophila carrying on...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to thank members of the Grillo-Hill pHly lab for discussions and support. We thank Tim Andriese, Randy Kirschner, Kitty (Ngoc-Huong) Nguyen, Marco Parent, Jonny Shaloub, and Librado Veliz for excellent technical support. This work was supported by NIH SC3GM132049 and 1R16GM153640 awards (BKGH), a CSU Biotechnology Faculty-Student Research Award (LM and BKGH), and start-up funds from the College of Science and the Department of Biological Sciences at San José State University (FJL). Special mention goes to Bernd Becker for their resourcefulness and assistance during this process. We thank the BioIcons (https://bioicons.com/) community for providing high-quality icons for our figures and especially to Serviere for the pipet icon, and DBCLS for the Drosophila, forceps, and desktop electron microscope icons used in Figure 1 and Figure 2, which are licensed under CC-BY 4.0 Unported. We also thank the SciDraw (https://scidraw.io/) community for providing high-quality icons for our figures, especially Diogo Losch De Oliveira (doi.org/10.5281/zenodo.3925953), which are licensed under Creative Commons 4.0 license (CC-BY).
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL serological pipette | ThermoFisher Scientific | 170353N | |
| 1.7 mL microcentrifuge tubes | Genesee Scientific | 24-282LR | |
| 20x Apo Microscope Objective | Mitutoyo Corp. | 378-804-3 | |
| Archival 65 lb cardstock | Neenah, Inc. | 91901 | |
| Canon EF 70-200 mm USM II telephoto lens | Canon | 3044C002 | |
| Canon EOS 6D Mark II DSLR Camera Body | Canon | 1897C002 | |
| Diffuser Dome | Macroscopic Solutions | PA-DIF-GIM-SM | |
| Diffuser for Mitutoyo M Plan APO Objectives | Macroscopic Solutions | mitutoyo-diffusers | |
| Drosophila vials and plugs | Genesee Scientific | 32-117BF | |
| Dumont #5 fine-tip forceps | Fisher Scientific | NC9889584 | |
| Goose feathers | Amazon | B01CMMJI6U | |
| Heavy-Duty Anodized Aluminum Tripod | Really Right Stuff, LLC | TFA-32G | |
| Kimwipes | Fisher Scientific | 06-666A | lint-free lab tissue |
| Levenhuk M1000 Plus Digital Camera | Levenhuk | 70358 | |
| No. 3 mounting pin | Indigo Instruments | 33414-3 | |
| Nutri-Fly Bloomington Drosophila media | Genesee Scientific | 66-113 | fly food |
| Point-Punch | M.C. Mieth Manufacturing, Inc. | 448Detail | |
| Screwknob Clamp | Really Right Stuff, LLC | SK-Clamp | For attaching the macro rail to the tripod |
| Stackshot Controller and Macro Rail | Cognisys Inc. | ST3X_100_BUNDLE | |
| Step-down Ring Adapter | RAF Camera | 763461174207 | Lens adapter to connect the microscope objective to the camera lens |
| Titebond Glue | Franklin International | 5013 | |
| Yongnuo YN-24-EX Macro Twin Lite Flash | Shenzhen Yongnuo Photographic Equipment Co. | YN-24EX | |
| Software | |||
| Canon EOS Utility (v. 3.16.1). | Canon | acquisition software | |
| FIJI | National Institutes of Health | Fiji is released as open source under the GNU General Public License. FIJI Version 2.14.0/1.54f | |
| GraphPad Prism | GraphPad Software, Boston, Massachusetts USA | Prism Version 10.3.1 | |
| Zerene Stacker (v.1.04) | Zerene Systems, LLC | Focus Stacking Software |
References
- Rubin, G. M., et al. Comparative genomics of the eukaryotes. Science. 287 (5461), 2204-2215 (2000).
- Ugur, B., Chen, K., Bellen, H. J. Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9 (3), 235-244 (2016).
- Hodgson, J. A., Parvy, J. -. P., Yu, Y., Vidal, M., Cordero, J. B. Drosophila larval models of invasive tumorigenesis for in vivo studies on tumour/peripheral host tssue interactions during cancer cachexia. Int J Mol Sci. 22 (15), 8317 (2021).
- Lam Wong, K. K., Verheyen, E. M. Metabolic reprogramming in cancer: mechanistic insights from Drosophila. Dis Model Mech. 14 (7), dmm048934 (2021).
- Bonini, N. M. A perspective on Drosophila genetics and its insight into human neurodegenerative disease. Front Mol Biosci. 9, e1060796 (2022).
- Drummond-Barbosa, D., Tennessen, J. M. Reclaiming Warburg: using developmental biology to gain insight into human metabolic diseases. Development. 147 (11), dev189340 (2020).
- Ready, D. F., Hanson, T. E., Benzer, S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 53 (2), 217-240 (1976).
- Johnson, R. I. Hexagonal patterning of the Drosophila eye. Dev Biol. 478, 173-182 (2021).
- Weasner, B. P., Kumar, J. P. The early history of the eye-antennal disc of Drosophila melanogaster. Genetics. 221 (1), iyac041 (2022).
- Pichaud, F., Casares, F. Shaping an optical dome: The size and shape of the insect compound eye. Semin Cell Dev Biol. 130, 37-44 (2022).
- Oster, I. I., Crang, R. E. Scanning electron microscopy of Drosophila mutant and wild type eyes. Trans Am Microsc Soc. 91 (4), 600-602 (1972).
- Gibb, T. J., Oseto, C. . Insect collection and identification: Techniques for the field and laboratory. , (2019).
- Mertens, J., Roie, M. V., Merckx, J., Dekoninck, W. The use of low cost compact cameras with focus stacking functionality in entomological digitization projects. ZooKeys. 712, 141-154 (2017).
- Brown, B. V. A further chemical alternative to critical-point-drying for preparing small (or large) flies. Fly Times. 11, 10 (1993).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Method. 9 (7), 676-682 (2012).
- Grillo-Hill, B. K., Choi, C., Jimenez-Vidal, M., Barber, D. L. Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife. 4, e03270 (2015).
- White, K. A., Grillo-Hill, B. K., Barber, D. L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 130 (4), 663-669 (2017).
- Peralta, J., et al. Drosophila Nhe2 overexpression induces autophagic cell death. Mol Biol Cell. 35 (7), br13 (2024).
- Munnik, C., Xaba, M. P., Malindisa, S. T., Russell, B. L., Sooklal, S. A. Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies. Front Genet. 13, 949241 (2022).
- Nitta, Y., Sugie, A. Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly. 16 (1), 275-298 (2022).
- Pletcher, R. C., et al. A genetic screen using the Drosophila melanogaster TRiP RNAi collection to identify metabolic enzymes required for eye development. G3: Genes|Genomes|Genetics. 9 (7), 2061-2070 (2019).
- Bangi, E., et al. A personalized platform identifies trametinib plus zoledronate for a patient with KRAS-mutant metastatic colorectal cancer. Sci Adv. 5 (5), eaav6528 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved