A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Alternative Strategy to Analyze In Vitro Cell Invasion of 3D Cultures
In This Article
Summary
Invasion is a major biological phenomenon in the development and progression of cancer. This process is influenced by non-neoplastic cells and components of the tumor microenvironment. The purpose of this study is to describe an alternative method for analyzing tumor cell invasion in vitro using three-dimensional (3D) cultures.
Abstract
Spheroid culture is a 3D model that provides an improved replication of the in vivo microenvironment compared to traditional two-dimensional (2D) cultures. Invasion is a cellular outcome of utmost interest in cancer biology. In this protocol, we have devised an alternative strategy for evaluating cancer cell invasion in vitro, employing heterospheroids comprised of oral squamous cell carcinoma (OSCC), cancer-associated fibroblasts (CAF), and monocytes. These heterospheroids aim to mimic the tumor microenvironment (TME), including two relevant non-neoplastic cell types alongside the cancer cells. Each cell type was labeled with vital fluorescent markers emitting in distinct wavelengths before spheroid formation. Once formed, heterospheroids were seeded onto a layer of human leiomyoma-derived extracellular matrix in the upper compartment of a microporous membrane. Invasion was assessed in the z-axis using confocal microscopy. Digital images were obtained in the corresponding fluorescent channels at 10 µm intervals, covering a depth of 90 µm in the z-axis. Analysis was performed using freeware image software by calculating the integrated fluorescence intensity in each image and fluorescence channel. This approach enables a more dynamic analysis of cell invasion patterns in a multilayered context, as well as the examination of spatial co-localization of different cell types during invasion.
Introduction
Invasion is a process in which neoplastic cells migrate through the extracellular matrix into the surrounding tissue1,2. As the tumor progresses, neoplastic cells are exposed to increasingly complex microenvironments. The tumor microenvironment includes various components of the extracellular matrix and non-neoplastic cell types. Stromal cells, including fibroblasts and resident macrophages, remodel the microenvironment by secreting extracellular matrix components and growth factors, and cytokines, which in turn influence neoplastic cell functions3. Stromal and infiltrating leukocyte cells also directly interact with neoplastic cells, with each other, and with the extracellular matrix in a three-dimensional microenvironment. Altogether, the TME composition influences the efficacy and pattern of neoplastic cell invasion4.
The intricate nature of the tumor microenvironment requires the implementation of efficacious methodologies for comprehending intercellular interactions within tumor tissue. Most in vitro assays used to assess invasion have primarily been conducted in traditional 2D models and/or single cell-type cultures5,6,7,8, which oversimplifies the complex process of invasion and has multiple limitations, such as the lack of tissue architecture, stromal components, and in vivo reproducibility. Spheroid cultures represent one model of in vitro 3D cultures that mimics tumor mass morphology, allowing for cell-cell and cell-matrix interactions involving physical and biochemical features9. The use of 3D culture models has grown in the last decades, and there is evidence that various biological outcomes, such as response to treatment, cell morphology, and gene expression, are different in comparison with the 2D model9,10.
This protocol demonstrates a method for assessing in vitro invasion in a human extracellular matrix using 3D spheroid cell culture. Moreover, we employed heterospheroids comprised of neoplastic cells, fibroblasts, and monocytes to mimic the tumor microenvironment with two relevant non-neoplastic cell types. The imaging of cells by confocal microscopy along different planes of invasion towards a microporous membrane enables the visualization of this process in a more dynamic and multilayered context.
Protocol
The use of primary human cells was approved by the Institutional Human Research Ethics Committee (CAAE 57895822.0.0000.5416) of the School of Dentistry at Araraquara - UNESP. The details of the reagents and the equipment used in the study are listed in the Table of Materials.
1. Preparation of cells for 3D cell culture
NOTE: All steps in this section must be performed within a laminar flow hood.
- Obtain single-cell suspensions of the cells that will form the heterospheroid.
NOTE: In this experiment, we generated spheroids (see step 2) consisting of OSCC cell line (SCC9), fibroblasts (primary cancer-associated fibroblast from tongue cancer biopsies), and monocytes (primary monocytes from healthy donors). Select the type of cells according to experimental interests. - Check the cell number and viability using a hemocytometer and trypan blue staining.
NOTE: The cell ratio in this protocol is 5:3:2 (5,000 neoplastic: 3,000 fibroblasts: 2,000 monocytes), totaling 10,000 cells per spheroid. Optimize the ratio and total number of cells according to experimental interests. - Separately dilute cells in 1.5 mL tubes with non-supplemented medium appropriate for each cell type (e.g., for neoplastic cells - DMEM/F12; for fibroblasts - DMEM; for monocytes - RPMI) to achieve an appropriate seeding density of 5,000 neoplastic cells per well, 3,000 fibroblasts per well, and 2,000 monocytes per well, or 500,000 cells per milliliter.
NOTE: To ensure an adequate number of cells for the experiment, use 20% more than the total required, considering potential losses during the staining and centrifugation steps. - Label the different cell types separately using distinct fluorescent vital stains that remain in living cells through several generations. Add optimized concentrations of fluorescent dye to the medium containing cells (e.g., neoplastic: CFSE - green dye; fibroblast: CMTPX - red dye; monocyte, Violet BMQC - blue dye) and incubate at 37 °C for 20 min.
NOTE: Select fluorescent probes with different excitation and emission spectra to avoid spectral overlap according to the specifications of the confocal microscope that will be used. - Centrifuge the cells at 400 x g for 5 min at room temperature to form a cell pellet. Aspirate the medium using a 200 µL pipette and re-suspend in 1 mL of 1x PBS.
- Centrifuge at 400 x g for 5 min to form a cell pellet and re-suspend the total number of cells in 1 mL of supplemented culture medium according to the cell type/cell line used (e.g., neoplastic - 100 µg/mL streptomycin, 100 IU/mL penicillin, 10% (vol) FBS, and 400 µg/mL hydrocortisone; fibroblast and monocytes - 100 µg/mL streptomycin, 100 IU/mL penicillin, 10% (vol) FBS). Check fluorescence intensity on the microscope and count the cells with trypan blue staining.
2. 3D cell culture
NOTE: This study employed the bioprinting magnetic field method11 to create heterospheroids. Here, cells are magnetized using biocompatible magnetic nanoparticles and subjected to gentle magnetic forces. Optimize the ratio of nanoparticles to cells according to the cell type. Alternative methods for establishing spheroid cultures may also be used. Conduct the steps in this section in a laminar flow hood.
- Add magnetic nanoparticles at a ratio of 1 µL of nanoparticles to 20,000 cells, and thoroughly re-suspend by mixing 10 times.
- Centrifuge the cells at 400 x g, room temperature, for 5 min, and re-suspend by mixing 10 times. Repeat this cycle of centrifuging/resuspension two additional times.
NOTE: Magnetized cells will form a brownish-colored pellet. - After completing the last centrifugation cycle, aspirate the medium and re-suspend the cells in 34 µL per well (34 µL/5,000 neoplastic cells; 34 µL/3,000 fibroblast cells; 34 µL/2,000 monocyte cells) of supplemented culture medium (DMEM/F12 containing 100 µg/mL streptomycin, 100 IU/mL penicillin, and 5% (vol) FBS) for the neoplastic cells.
NOTE: Calculate the volume based on the well size. This protocol utilized 96-well plates, totaling 100 µL of medium per well. - Mix all the cells into a new tube for co-culturing, and then re-suspend.
- Transfer 100 µL of co-cultured cells into each well of an ultra-low attachment plate.
- Insert the spheroid drive magnetic field under the plate to induce aggregation and spheroid formation, and place it in the incubator (5% CO2, 37 °C) for 3 h.
NOTE: The time of exposure to the magnetic field can vary according to spheroid size or composition. - Remove the magnetic field and place the plate in the incubator (5% CO2, 37 °C) for at least 24 h to allow the production of extracellular matrix proteins and cell-to-cell/cell-to-matrix interactions.
3. Preparation of extracellular matrix-coated microporous membrane
NOTE: Conduct the steps in this section in a laminar flow hood.
- Add 50 µL of the commercially available extracellular matrix to the center of a 24-well microporous membrane using a 200 µL tip.
NOTE: Keep the extracellular matrix on wet ice. Do not touch the microporous membrane when placing the matrix on top. - Remove bubbles using a sterile needle and ensure that the matrix covers the entire membrane surface.
- Place the membrane in the incubator (37 °C) for 1 h to allow gel formation of the extracellular matrix.
4. Invasion assay
NOTE: Conduct the steps in this section in a laminar flow hood.
- Add 500 µL of DMEM/F12 culture medium supplemented with 10% fetal bovine serum to the lower chamber of the microporous membrane as a chemoattractant.
- Carefully remove the medium from the formed spheroids and gently add 50 µL/well of fresh non-supplemented DMEM/F12 medium over the heterospheroid.
NOTE: This step can be performed using a holding drive for the bioprinting magnetic field method. - Cut the edge of a 200 µL low retention tip with a sterile scissors.
NOTE: The size of the tip will depend on the size of the spheroid. - Collect the spheroid and 50 µL of medium with the low retention tip.
NOTE: Perform this step slowly and carefully to avoid disaggregation of the spheroid. - Place the spheroid gently on top of the extracellular matrix.
NOTE: Do not touch the jellified extracellular matrix. - Carefully add 150 µL of non-supplemented DMEM/F12 medium drop by drop to reach a total volume of 200 µL.
- Place the plate in the incubator (5% CO2, 37 °C) for 48 h.
NOTE: The time of incubation can vary depending on the cell line or protocol.
5. Image acquisition
- Use a confocal microscope for imaging.
- Set up the microscope based on the excitation and emission characteristics of vital fluorescent stains used (e.g., CFSE dye, 488 nm laser, and 520 nm detection wavelength; fibroblast: CMTPX dye, 561 nm laser, and 610 nm detection wavelength; monocyte, Violet BMQC dye, 405 nm laser, and 516 nm detection wavelength).
- Load the plate onto the microscope stage.
- Locate spheroids/cells using the bright field at 10x magnification.
- Adjust the zoom to 40x magnification.
- Set laser power and detector gain for each fluorescent channel.
- Identify the Z-Stack starting point as the membrane focal plane by locating the membrane pores using the bright field.
- Capture three images below and five to eight images above the membrane along the z-axis for each channel, including the bright field. Ensure 10 µm intervals between images, covering a total depth of 90-120 µm in the z-axis.
- Save the project as a .czi extension file after generating the images.
6. Image extraction
NOTE: The usage of image software is explained below.
- Open the .czi file using the image editing software (see Table of Materials).
- Click on Processing in the top right corner, then select Single. Next, click on Method and choose Image Export.
- Select the file under Input.
- Click on the Parameters button, then select the following settings: Filetype (Tagged Image File Format - .TIFF), Compression (LZM), Resize (100%), Apply Display Curve And Channel Color, Individual Channel Images, Use Channel Names, Define Subsets (Channel, select all channels), Z-Position (extract all), Region (Full), Tiles (Export selected tiles) and Export to (select the file of destination).
NOTE: To extract merged channel images, select the following settings: Filetype (Tagged Image File Format - .TIFF), Compression (LZM), Resize (100%), Apply Display Curve And Channel Color, Merged Channels Image, Use Channel Names, Define Subsets (Channel, select the specific channels to overlay), Z-Position (extract all), Region (Full), Tiles (Export selected tiles) and Export to (select the file of destination). - Click on the Apply icon located in the top right corner.
NOTE: This step will simultaneously export images from the different channels.
7. Image analysis
NOTE: The usage of free software is explained in this step.
- Click on File in the top left corner, select Open, choose the image, and then click on OK.
- Click on Analyze, then Set Measurements, and select Area, Integrated Density, and Display Label. Click on OK.
- Click on Analyze, and then Measure.
NOTE: Ensure the area of all images is the same. "Integrated Density" displays two values: (1) Raw Integrated Density (RawIntDen): Sum of all pixel values in the image; (2) Integrated Density (IntDen): Product of Mean Gray Value and Area. Since the image is RGB color and the area of all images is the same, consider using RawIntDen as the fluorescence measurement value. Differences in fluorescence values (intensity and quantity) among images may indicate variations in cell invasion and proliferation intensity. Another method to normalize the invasion pattern in the extracellular matrix toward the microporous membrane using co-cultures of different cell types is to calculate the ratio of RawIntDen values:
Ratio RawIntDen =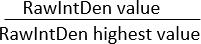
A ratio of 1 represents the image/location along the z-axis with the highest invasion/localization of cells for that specific cell type.
Results
This study presents an alternative method for assessing cell invasion in vitro using spheroids composed of different cell types. It enables the analysis of cell invasion patterns in a multilayered context and the examination of cell co-localization dynamics during invasion (Figure 1).
The bright-field images represent spheroid/cell localization, identified by dark/black agglomerates (Figure 2A). The plane of the membrane is d...
Discussion
Three-dimensional spheroid cultures are a powerful approach to investigate various aspects of cell biology. In this study, we report a protocol for evaluating neoplastic cell invasion using heterospheroids that mimic the tumor microenvironment in a multilayered and more dynamic approach. This method allows for the assessment of co-localization and patterns of invasion in the presence of non-neoplastic stromal cells.
There are some critical steps that warrant consideration. First, it is importa...
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the São Paulo Research Foundation (FAPESP 20/10544-1 and 20/10664-7). Some images were generated using BioRender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 96 Well Bioprinting Kit | Greiner Bio-one | 655840 | Spheroid drive magnetic field. Holding drive. 96 well microplate, cell-repellent surface |
| CellTrace CFSE Cell Proliferation Kit | Invitrogen, ThermoFisher Scientific | C34554 | |
| CellTracker Red CMTPX | Invitrogen, ThermoFisher Scientific | C34552 | |
| CellTracker Violet BMQC Dye | Invitrogen, Thermo Fisher | C10094 | |
| DMEM | Gibco, ThermoFisher Scientific | 31600-034 | |
| DMEM/F12 | Gibco, ThermoFisher Scientific | 12400-024 | |
| Extracellular matrix with Myogel | doi: 10.1186/s12885-015-1944-z | Mix composed of myogel (2.4 mg/mL), collagen (0.8 mg/mL) and non-supplemented medium of neoplastic cell | |
| FBS | Gibco, ThermoFisher Scientific | 12657-029 | |
| Hydrocortisone | Sigma | H088 | |
| ImageJ | National Institutes of Health | Software | |
| Nanoshuttle-PL | Greiner Bio-one | 657841 | Magnetic nanoparticles |
| Needle 23 G | Medix | AHMD004 | 25 x 0.60 mm (23 G x 1) |
| PBS pH 7.2 (1x) | Gibco, ThermoFisher Scientific | 2012-027 | |
| Penicillin-Streptomycin (5,000 U/mL) | Gibco, ThermoFisher Scientific | 15070063 | |
| Pipet Tips | Axygen, Corning Inc. | TF-200-L-R-S | Pipet tip with barrier (filter), 200 µL Low Retention Filter, short, maximum recovery |
| RPMI | Gibco, ThermoFisher Scientific | 31800-022 | |
| TransWell 24 well | Costar, Corning Inc. | 3422 | Transwell Permeable Supports 6.5 mm Insert, 24 well plate; 0.8 µm Polycarbonate Membrane |
| Zen 3.4 (blue edition) | Zeiss Group | Software |
References
- Steeg, P. S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 12 (8), 895-904 (2006).
- Friedl, P., Wolf, K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 3, 362-374 (2003).
- Friedl, P., Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 147 (5), 992-1009 (2011).
- Wu, J. S., et al. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl Oncol. 14 (1), 100899 (2021).
- Pijuan, J., et al. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis. Front Cell Dev Biol. 7, 1-16 (2019).
- Nagy, &. #. 1. 9. 3. ;. G., Székács, I., Bonyár, A., Horvath, R. Simple and automatic monitoring of cancer cell invasion into an epithelial monolayer using label-free holographic microscopy. Sci Rep. 12 (1), 1-13 (2022).
- Kramer, N., et al. In vitro cell migration and invasion assays. Mutat Res Rev Mutat Res. 752 (1), 10-24 (2013).
- Justus, C. R., Leffler, N., Ruiz-Echevarria, M., Yang, L. V. In vitro cell migration and invasion assays. J Vis Exp. 88, e51046 (2014).
- Kapałczyńska, M., et al. 2D and 3D cell cultures - A comparison of different. Arch Med Sci. 14 (4), 910-919 (2016).
- Cacciamali, A., Villa, R., Dotti, S. 3D cell cultures: Evolution of an ancient tool for new applications. Front Physiol. 13, 1-15 (2022).
- Trindade, J., et al. Magnetic 3D cell culture State of the art and current advances. Life Sci. 286, 1-8 (2021).
- Han, S. J., Kwon, S., Kim, K. S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 21 (1), 1-19 (2021).
- Lin, Y. N., et al. Monitoring cancer cell invasion and T-cell cytotoxicity in 3D culture. J Vis Exp. 160, e61392 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved