Ideal Gas Law
Source: Laboratory of Dr. Andreas Züttel - Swiss Federal Laboratories for Materials Science and Technology
The ideal gas law describes the behavior of most common gases at near-ambient conditions and the tendency of all chemical matter in the dilute limit. It is a fundamental relationship between three measurable macroscopic system variables (pressure, temperature, and volume) and the number of molecules of gas in the system, and is therefore an essential link between the microscopic and the macroscopic universes.
The history of the ideal gas law dates to the middle of the 17th century when the relationship between the pressure and volume of air was found to be inversely proportional, an expression confirmed by Robert Boyle and which we now refer to as Boyle’s law (Equation 1).
P 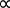 V-1 (Equation 1)
V-1 (Equation 1)
Unpublished work by Jacques Charles in the 1780s, which was extended to numerous gases and vapors by Joseph Louis Gay-Lussac and reported in 1802, established the directly proportional relationship between the absolute temperature and volume of a gas. This relationship is called Charles's law (Equation 2).
V 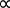 T (Equation 2)
T (Equation 2)
Guillaume Amontons is typically credited with first discovering the relationship between the temperature and pressure of air within a fixed volume at the turn of the 18th century. This law was also extended to numerous other gases by Joseph Louis Gay-Lussac at the beginning of the 19th century and is therefore either referred to as either Amontons’s law or Gay-Lussac's law, stated as shown in Equation 3.
P 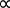 T (Equation 3)
T (Equation 3)
Together, these three relationships can be combined to give the relationship in Equation 4.
V 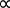 T (Equation 4)
T (Equation 4)
Finally, in 1811, it was proposed by Amedeo Avogadro that any two gases, held in the same volume and at the same temperature and pressure, contain the same number of molecules. This led to the conclusion that all gases may be described by a common constant, the ideal gas constant R, that is independent of the nature of the gas. This is known as the ideal gas law (Equation 5).1,2
PV 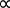 T (Equation 5)
T (Equation 5)
1. Measuring the Volume of the Sample
- Clean the sample carefully and dry it.
- Fill a high-resolution graduated cylinder with enough distilled water to cover the sample. Note the initial volume
- Drop the sample into the water and note the volume change. This is the volume of the sample, V.
- Remove the sample and dry it. Note: alternatively, measured the side length(s) of the sample and calculate its volume using geometry.
2. Load the Sample in th
The ideal gas law is a valid description of the actual gas properties of numerous common gases at conditions near ambient (Figure 1 inset) and is therefore useful in the context of many applications. The limitations of the ideal gas law in describing systems under conditions of high pressures or low temperatures can be explained by the increasing importance of molecular interactions and/or the finite size of the gas molecules contributing to the system’s properties. Therefore, gases with
The ideal gas law is such a fundamental equation of the chemical sciences that it has a plethora of uses both in day-to-day laboratory activities as well as in calculations and modeling of even highly complex systems, at least to first approximation. Its applicability is limited only by the approximations inherent to the law itself; at near-ambient pressures and temperatures, where the ideal gas law is well valid for many common gases, it is widely employed in the interpretation of gas-based systems and processes. Two ex
- Zumdahl, S.S., Chemical Principles. Houghton Mifflin, New York, NY. (2002).
- Kotz, J., Treichel, P., Townsend, J. Chemistry and Chemical Reactivity. 8th ed. Brooks/Cole, Belmont, CA (2012).
- Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications.Academic Press, San Diego, CA. (2014).
Vai a...
Video da questa raccolta:

Now Playing
Ideal Gas Law
General Chemistry
77.8K Visualizzazioni

Common Lab Glassware and Uses
General Chemistry
652.7K Visualizzazioni

Solutions and Concentrations
General Chemistry
272.6K Visualizzazioni

Determining the Density of a Solid and Liquid
General Chemistry
554.3K Visualizzazioni

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
382.9K Visualizzazioni

Determining the Empirical Formula
General Chemistry
179.1K Visualizzazioni

Determining the Solubility Rules of Ionic Compounds
General Chemistry
140.9K Visualizzazioni

Using a pH Meter
General Chemistry
344.1K Visualizzazioni

Introduction to Titration
General Chemistry
423.0K Visualizzazioni

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.2K Visualizzazioni

Le Châtelier's Principle
General Chemistry
263.7K Visualizzazioni

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.3K Visualizzazioni

Determining Rate Laws and the Order of Reaction
General Chemistry
195.6K Visualizzazioni

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Visualizzazioni

Coordination Chemistry Complexes
General Chemistry
91.1K Visualizzazioni
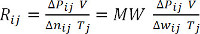 (Equation 9)
(Equation 9)