Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
Panoramica
Source: Laboratory of Dr. Terry Tritt — Clemson University
Differential Scanning Calorimetry (DSC) is a method of thermodynamic analysis based on heat-flux method, wherein a sample material (enclosed in a pan) and an empty reference pan are subjected to identical temperature conditions. The energy difference that is required to maintain both the pans at the same temperature, owing to the difference in the heat capacities of the sample and the reference pan, is recorded as a function of temperature. This energy released or absorbed is a measure of the enthalpy change (ΔΗ) of the sample with respect to the reference pan.
Procedura
1. Baseline Measurement
- Controller, Measuring unit, Computer system, Thermostat approximately 60 min. before starting the measurement. Purge gases must be connected to the system.
- Place two empty crucibles (with lid) into the sample carrier. The crucible material may be chosen based on the temperature range to be measured.
- Move the furnace to measuring position.
- Adjust the measuring conditions (gas, vacuum).
- Start the measurement program.
- Proceed to create a b
Risultati
ZnO formation via Decomposition of ZnCO3
The change in enthalpy per degree, at constant pressure is equivalent to the heat capacity of a material at constant pressure given by Equation 1. The enthalpy change is obtained by estimating the area under the curve between two temperature limits given by Equation 2.
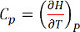 (Equation 1)
(Equation 1)
Applicazione e Riepilogo
A major application area of DSC is the glass transition (Tg) in amorphous polymers, in which the material changes from a rigid glassy state to a viscous liquid state. Pharmaceutical research on nano-particles is also an emerging field, where the DSC has been used to quantify amorphous or crystalline phase in nano-solids. A review of DSC techniques on applications in biology and nano-science has been provided by Gill et al.3 Nanostructured lipid carriers (NLC) have potential applications in
Riferimenti
- Robinson, J.W., Skelly Frame, E.M., Frame, GM. Undergraduate Instrumental Analysis. Marcel Decker, New York, NY. (2005).
- Liu, S., Li, C., Yu, J., Xiang, Q., Improved visible-light photocatalytic activity or porous carbon self-doped ZnO nanosheet-assembled flowers. CrystEngComm. 13, p 2533 (2011).
- Gill, P., Tohidu Moghadam, T., Ranjbar, B. Differential Scanning Calorimetry Techniques: Applications in Biology and Nanoscience. Biomolecular Techniques. 21, 167-193 (2010).
Vai a...
Video da questa raccolta:

Now Playing
Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Visualizzazioni

Common Lab Glassware and Uses
General Chemistry
653.1K Visualizzazioni

Solutions and Concentrations
General Chemistry
272.9K Visualizzazioni

Determining the Density of a Solid and Liquid
General Chemistry
554.6K Visualizzazioni

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.0K Visualizzazioni

Determining the Empirical Formula
General Chemistry
179.9K Visualizzazioni

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.0K Visualizzazioni

Using a pH Meter
General Chemistry
344.2K Visualizzazioni

Introduction to Titration
General Chemistry
423.3K Visualizzazioni

Ideal Gas Law
General Chemistry
78.0K Visualizzazioni

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.2K Visualizzazioni

Le Châtelier's Principle
General Chemistry
263.9K Visualizzazioni

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.4K Visualizzazioni

Determining Rate Laws and the Order of Reaction
General Chemistry
195.7K Visualizzazioni

Coordination Chemistry Complexes
General Chemistry
91.2K Visualizzazioni