Preparing Anhydrous Reagents and Equipment
Source: Laboratory of Dr. Dana Lashley - College of William and Mary
Demonstrated by: Timothy Beck and Lucas Arney
Many reactions in organic chemistry are moisture-sensitive and must be carried out under careful exclusion of water. In these cases the reagents have a high affinity to react with water from the atmosphere and if left exposed the desired reaction will not take place or give poor yields, because the reactants are chemically altered.
In order to prevent undesired reactions with H2O these reactions have to be carried out under an inert atmosphere. An inert atmosphere is generated by running the reaction under nitrogen gas, or in more sensitive cases, under a noble gas such as argon.
Every component in such a reaction must be completely anhydrous, or free of water. This includes all reagents and solvents used as well as all glassware and equipment that will come into contact with the reagents. Extremely water-sensitive reactions must be carried out inside of a glovebox which provides a completely sealed off anhydrous environment to work under via a pair of gloves which protrudes out to one of the sides of the chamber.
Drying of Glassware
1. Oven-Drying
- Remove all pieces that are not made of glass, such as the stopcock of an addition funnel.
- Place all glassware that are part of the apparatus in a drying-oven set to about 125 °C for at least 24 h before use.
- Put on heat protection gloves and remove glassware from the oven. Be very careful when handling hot glass while assembling the apparatus.
- For best results flush the apparatus with an inert gas such as N2&#
A classical example for a reaction that must be done under anhydrous conditions is the Grignard reaction. (Equation 1)
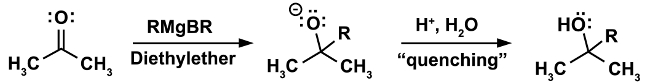
In the first step of the reaction, the nucleophilic attack of the Grignard reagent RMgX occurs on an electrophile (in this case a ketone). In this step
- Burfield, D. R. and Smithers, R. H. Desiccant efficiency in solvent and reagent drying. 7. Alcohols. J. Org. Chem. 48 (14), 2420-2422 (1983).
- Williams, D. B. G. and Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 75 (24), 8351-8354 (2010).
スキップ先...
このコレクションのビデオ:

Now Playing
Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.1K 閲覧数

Introduction to Catalysis
Organic Chemistry
34.1K 閲覧数

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
166.2K 閲覧数

Conducting Reactions Below Room Temperature
Organic Chemistry
70.3K 閲覧数

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.5K 閲覧数

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
55.9K 閲覧数

Purifying Compounds by Recrystallization
Organic Chemistry
705.0K 閲覧数

Separation of Mixtures via Precipitation
Organic Chemistry
157.2K 閲覧数

Solid-Liquid Extraction
Organic Chemistry
236.9K 閲覧数

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.3K 閲覧数

Fractional Distillation
Organic Chemistry
332.7K 閲覧数

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.3K 閲覧数

Performing 1D Thin Layer Chromatography
Organic Chemistry
288.3K 閲覧数

Column Chromatography
Organic Chemistry
358.3K 閲覧数

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
246.7K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved