A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microbiota of Attine Ants
In This Article
Summary
We propose an optimized Scanning Electron Microscopy protocol for visualizing highly heterogeneous and delicate samples containing plant and fungal biomass, together with microbiota and biofilm. This protocol allows describing the spatial dimensions of the microbiota organization.
Abstract
In macroscale ecosystems, such as rainforests or coral reefs, the spatial localization of organisms is the basis of our understanding of community ecology. In the microbial world, likewise, microscale ecosystems are far from a random and homogeneous mixture of organisms and habitats. Accessing the spatial distribution of microbes is fundamental for understanding the functioning and ecology of the microbiota, as cohabiting species are more likely to interact and influence each other's physiology.
An interkingdom microbial ecosystem is at the core of fungus-growing ant colonies, which cultivate basidiomycete fungi as a nutritional resource. Attine ants forage for diverse substrates (mostly plant-based), metabolized by the cultivated fungus while forming a spongy structure, a "microbial garden" that acts as an external gut. The garden is an intertwined mesh of fungal hyphae growing by metabolizing the substrate, opening niches for a characteristic and adapted microbiota to establish. The microbiota is thought to be a contributor to substrate degradation and fungal growth, though its spatial organization is yet to be determined.
Here, we describe how we employ Scanning Electron Microscopy (SEM) to investigate, with unprecedented detail, the microbiota and biofilm spatial organization across different fungiculture systems of fungus-growing ants. SEM imaging has provided a description of the microbiota spatial structure and organization. SEM revealed that microbiota commonly assemble in biofilms, a widespread structure of the microbial landscapes in fungiculture. We present the protocols employed to fix, dehydrate, dry, sputter coating, and image such a complex community. These protocols were optimized to deal with delicate and heterogeneous samples, comprising plant and fungal biomass, as well as the microbiota and the biofilm.
Introduction
Ecosystems are composed of organisms interconnected by processes in a specific geographical location (i.e., the environment). Organisms interact with their environment over time, from which complex and heterogeneous spatial patterns emerge. Spatial patterning determines ecological diversity and stability and, ultimately, ecosystem functioning1,2,3,4. In macroscale ecosystems, such as wetlands, savannas, coral reefs, and arid ecosystems, spatial patterns are correlated with resource flow and concentration. Permitting resource optimization, spatial heterogeneity and patterning result in more resilient ecosystems than homogeneous ones2. The spatial localization of organisms being at the basis of community ecology is also translated to the microbial world.
Microbial ecosystems, far from organisms randomly and homogeneously mixed throughout microhabitats, exhibit spatial patterns defining much of their functioning5,6,7. From Winogradsky columns to environmental- and host-associated microbiota, these ecosystems are heterogeneously organized in space, with spatial arrangements eliciting different phenotypic responses. Cohabiting species are more likely to interact and influence each other's physiology. Thus, the community spatial organization, more than its composition per se, delimits ecosystem properties and ecological niches5,7,8. Illustrating these concepts, changes in spatial patterning seem correlated to the pathological progression of dental plaques, caries, gum diseases9,10, inflammatory bowel disease11, cystic fibrosis lung infections, chronic wound infections12,13, colorectal cancer, and adenomas14.
Under the scope of microbial biogeography (the study of biodiversity distribution and patterning across space and time on a microscale), the knowledge of microbial ecosystems is vastly benefitted by comprehending their spatial patterns6,13,15,16,17. We have looked into spatial patterns of an insect-built microbial ecosystem, found at the core of the charismatic fungus-growing attine ant (Hymenoptera: Formicidae: Myrmicinae: Attini: Attina) colonies. There resides a "microbial garden," centered around a basidiomycete fungus in the tribe Leucocoprinae (Basidiomycota: Agaricaceae) or in the family Pterulaceae (Basidiomycota: Agaricales)18,19,20,21,22. The garden is a spongy structure emerging from an intertwined mesh of hyphae that grows by metabolizing the mostly plant-based substrate incorporated by the ants (Figure 1). These may include, according to the attine genera: dry plant parts, insect frass and carcasses, freshly cut leaves, seeds, and floral parts23,24. Analogous to an external herbivorous gut, the garden enzymatically and chemically converts recalcitrant polymers into labile nutritional resources, providing the ants with essential amino acids, lipids, and soluble sugars21,25,26,27,28.
Ultrastructural, enzymatic, and transcriptomic analyses carried out for gardens of the leaf-cutting genera Atta and Acromyrmex suggest these environments structure a continuum of substrate degradation and nutritional patches26,29,30,31,32. Young parts of the garden tend to be darker due to freshly incorporated substrate after being fragmented. These recently added substrates are often colonized from the edges, which were cut by ant workers and inoculated with mycelial clumps. Radiating from cut edges, fungal hyphae spread over the substrate29,32,33. Hyphal abundance increases as substrate degradation progresses, resulting in whitish and metabolically active regions30,31,32. Older regions, with more degraded substrate and an abundant microbiota29,32, tend to present brownish tones and higher humidity. Workers remove fragments of this region, separating them in waste piles, where they also take substrates that harm the fungal symbiont34,35,36. Waste piles, although physically detached from the garden, are a spot of continuous substrate degradation and nutrient cycling by the abundant inhabitant microbiota29,32,37,38,39.
A microbiota mainly composed of Enterobacter, Klebsiella, Pantoea, Pseudomonas, and Serratia, also inhabits the garden, apparently being shared by diverse attine fungiculture systems. Encoding metabolic pathways that could complement fungal metabolism, the microbiota potentially participates in the garden's physiological responses40,41,42,43,44. Not only did metagenomic data indicate that the microbiota was there41,42, but also Scanning Electron Microscopy (SEM) analysis of leaf-cutting ants' fungiculture showed mostly rod-shaped bacteria over plant substrate32. Though bacteria (including cellulolytic strains) were isolated from the entire garden, they were visualized only in older parts of the garden and in waste piles, as well as in the initial pellet carried by foundress queens29,32. It was also uncertain whether the microbiota could form biofilms in vivo (i.e., in the garden and waste), as suggested by their metabolic capacity42 and observed in vitro44.
Here, we employed SEM to further comprehend the microbiota spatial organization across the garden regions, detailing microbiota-substrate and microbiota-hyphae physical interactions. By providing images with larger focal depth, SEM permits observations of three-dimensional microscopic structures in high resolution, enabling a thorough analysis of the garden microbiota spatial patterns. We detail steps to fix, dehydrate, dry, sputter coat, and image such heterogeneous and delicate fungal-based samples. By removing the postfixation step using osmium tetroxide (OsO4) and reducing the dehydration time, we simplified the protocols32,33,45 for preparing garden and waste samples for SEM analysis. This adapted protocol preserves hyphal structural patterns, as well as the microbiota and biofilm spatial organization, and could be applied to other delicate microbial ecosystems and biofilms.

Figure 1: Attine microbial gardens. The garden is a sponge-like structure resulting from an intertwined mesh of hyphae that grows by metabolizing the mostly plant-based substrate incorporated by the ants. Also inhabiting the garden is the microbiota, which encodes metabolic pathways that could complement fungal metabolism. Metagenomic data and previous Scanning Electron Microscopy analysis indicated its presence, though we had scarce knowledge of its spatial organization and physical interactions with the substrate and fungal hyphae. We employed SEM to unveil the microbiota and biofilm spatial organization and patterning. Illustrations by Mariana Barcoto (garden and microbiota adapted from Barcoto and Rodrigues 94), and photos by Mariana Barcoto and Enzo Sorrentino. Please click here to view a larger version of this figure.
Protocol
1. Sampling field colonies
NOTE: When collecting ant colonies, certify that all the permissions required by local legislation are obtained before collecting. In our case, the collecting permit #74585 was issued by Instituto Chico Mendes de Conservação e Biodiversidade (ICMBio). When the samples come from a lab colony, go to section 2.
- Locate and mark the colony. Excavate a trench surrounding the nest area until the garden chamber is exposed (Figure 2A).
NOTE: Some attine species may build their colonies under the leaf litter or inside rotten logs. In such cases, carefully revolve the litter or carefully break the logs for collecting the samples. For detailed information on locating, collecting, and maintaining living colonies of diverse attine ant species, see Sosa-Calvo et al.46. - Open the garden chamber laterally to prevent the soil from falling on the garden surface. Carefully collect garden samples using either entomological forceps, a spoon, or a kitchen skimmer, depending on the garden size.
NOTE: Be sure to sterilize the tools before collecting. When collecting leaf-cutting ants' gardens, wear thick fabric gloves to prevent (or at least attenuate) worker bites. For other attine species, fabric gloves are optional. - Transfer the garden samples to a clean plastic recipient containing a layer of plaster for balancing the garden's humidity. After transferring the garden and ant workers, hermetically close the recipient to avoid sample drying. Store garden samples at 23-25 °C until processing.
- Close the trench with previously removed soil.
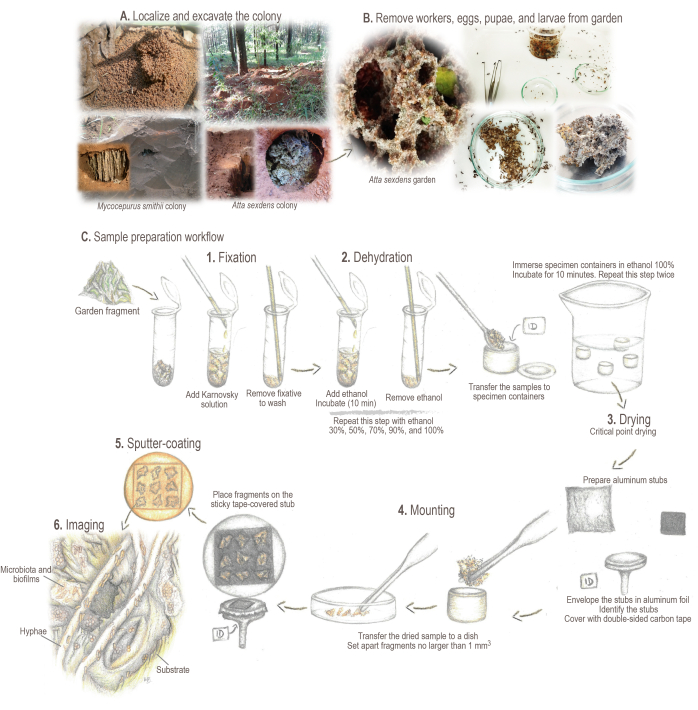
Figure 2: Sample preparation protocol. (A) Sampling of field colonies. (B) Sample processing. (C) Brief fundamentals and workflow for sample preparation: 1. Fixation: for strengthening and preserving sample structure. 2. Dehydration: samples' water content is exchanged for ethanol. 3. Critical point drying: liquid CO2 replaces ethanol and is evaporated. 4. Mounting: sample displayed for analysis. 5. Sputter-coating with gold: prevent sample charging. 6. Imaging. Illustrations and photos by Mariana Barcoto. Please click here to view a larger version of this figure.
2. Reagents
NOTE: Bear in mind that the following solutions should be prepared beforehand.
- Prepare 0.2 M sodium cacodylate buffer. For this, dissolve 42.8 g of sodium cacodylate in 800 mL of distilled water, stirring until dissolution and pH stabilization at 7.2 (if required, adjust pH using hydrochloric acid). Make up the volume of the solution to 1 L by adding distilled water. Store the solution at 4 °C (for ~1 month).
CAUTION: Glutaraldehyde and paraformaldehyde are toxic and should be handled within a fume hood. Wear nitrile gloves and protective glasses all the time while handling such reagents. - Prepare Karnovsky's fixative (modified from Karnovsky)47. For this, add 10 mL of 25% glutaraldehyde aqueous solution and 10 mL of 20% paraformaldehyde aqueous solution to 25 mL of 0.2 M sodium cacodylate buffer and mix. Add 1 mL of 0.1 M calcium chloride (CaCl2) and make up the volume to 100 mL by adding distilled water. Store the solution at 4 °C (for up to 1 month).
CAUTION: Karnovsky's fixative is harmful when inhaled and may cause skin and eye irritation. Thus, avoid breathing vapors, using only outdoors or in a well-ventilated area. Wear nitrile gloves and protective glasses all the time when handling the solution. - Using absolute ethanol (analytical grade), prepare 30%, 50%, 70%, and 90% ethanol solutions in distilled water; 100% ethanol is also required.
3. Sample fixation
NOTE: Fixatives harden and preserve samples, maintaining morphological features. Aldehydes (such as paraformaldehyde and glutaraldehyde) are non-coagulant fixatives of cross-linking type, inducing cross-links within and between proteins and nucleic acids48.
- Remove workers, eggs, pupae, and larvae from the garden samples using entomological forceps (Figure 2B). Set apart garden fragments no larger than 5 mm3. Add the fragments to a 2 mL tube (Figure 2C).
- To the tubes containing the samples, use a Pasteur glass pipette to add ~1 mL of Karnovsky fixative solution (ensure the sample is completely covered). Mix by gentle agitation to help the sample to soak and incubate at 4 °C for at least 24 h before continuing sample processing (Figure 2C.1).
NOTE: When the following dehydration steps will not be carried out immediately after the fixation, the protocol may be paused at this step, and samples may be stored for ~1 year at 4 °C.
We suggest using a Pasteur glass pipette, as it is composed of an inert material and is easier to clean for reuse afterward. Garden material is highly hydrophobic, tending to float over the surface of the fixative solution. It usually takes up to 5 min to be completely soaked in the fixative. Make sure the fixative covers the garden fragments since its volume tends to be reduced as it enters the sample's pores. We empirically verified that after the sample was soaked and became wet, its components (in particular, the delicate fungal mycelium) became susceptible to shattering when mixed further. Thus, we recommend avoiding shaking the samples as much as possible.
4. Sample dehydration
NOTE: The ethanol washing series gradually exchanges the water in samples for ethanol. It is important to start with a low-concentration ethanol solution (see below) to avoid excessively damaging or collapsing such delicate samples49.
- Completely remove Karnovsky's fixative solution using a glass pipette, being careful to not disrupt the sample (Figure 2C.1).
NOTE: Discard Karnovsky's fixative in a recipient properly labeled for toxic chemical residue management. - Immediately after removing the fixative, add 1 mL of 30% ethanol, being careful to not disrupt the sample, and incubate for 10 min at room temperature (Figure 2C.2).
NOTE: Samples need to always be soaked in solution. Make sure to quickly replace solutions during the ethanol gradual washing series. As the garden loses its porous aspect and aggregates at the tube bottom, 1 mL of ethanol tends to be enough to cover the sample (when the sample is no larger than 5 mm3). However, if the samples are not completely covered, add ethanol until the sample is completely covered. - Completely remove 30% ethanol with a glass pipette, being careful not to disrupt the sample. Discard 30% ethanol properly.
NOTE: For the entire ethanol gradual washing series, discard ethanol in a recipient properly labeled for toxic chemical residue management. - Add 1 mL of 50% ethanol and incubate for 10 min at room temperature. Completely remove 50% ethanol with a glass pipette, being careful not to disrupt the sample. Discard 50% ethanol properly.
- Add 1 mL of 70% ethanol and incubate for 10 min at room temperature. Completely remove 70% ethanol with a glass pipette, being careful not to disrupt the sample. Discard 70% ethanol properly.
NOTE: At the 70% ethanol washing step, the user may pause the protocol if needed, as sample tubes may be stored overnight at 4 °C, when the material will not be immediately processed. - Add 1 mL of 90% ethanol and incubate for 10 min at room temperature. Completely remove 90% ethanol with a glass pipette, being careful not to disrupt the sample. Discard 90% ethanol properly.
- Add 1 mL of 100% ethanol and incubate for 10 min at room temperature. Completely remove 100% ethanol with a glass pipette, being careful to not disrupt the sample. Discard 100% ethanol properly.
- Using a forceps and/or a spatula, carefully transfer the samples to specimen containers for the critical point dryer (CPD), containing sample identification labels (made previously in paper and pencil). For avoiding samples drying, this transfer is carried out with the container placed in a Petri dish covered with 100% ethanol.
- Put the lids on the containers and immerse them in a graduated glass beaker containing 100% ethanol enough to submerge the containers. Cover the glass beaker and incubate for 10 min at room temperature; then properly discard 100% ethanol.
- Transfer the specimen containers to another graduated glass beaker containing enough 100% ethanol to submerge the containers. Cover the glass beaker and incubate for 10 min at room temperature; then, transfer specimen containers to the critical point dryer.
NOTE: After the washing series, glass pipettes and beakers should be abundantly rinsed with distilled water, and this residual water should be discarded in a recipient properly labeled for toxic chemical residue management. After rinsing, glass items may be washed with neutral detergent, rinsed with tap water, and air dried.
5. Critical point drying
NOTE: A Critical Point Dryer exchanges the ethanol in samples for liquid carbon dioxide (CO2), which evaporates from the sample at higher temperature and pressure. Please follow the manufacturer's instructions for such procedures.
- Switch on the equipment.
- Open the chamber, place the sample containers inside, and add 100% ethanol until it covers the containers. Close the chamber.
- Activate the Cool option, and wait until the temperature reaches 10 °C.
- Open the CO2 cylinder valve and activate the Stirrer option.
- Activate the CO2 in option, always checking the chamber to verify how much CO2 has already filled it. When the chamber is almost filled, deactivate the CO2 in option and activate the Exchange option, keeping it activated until there is enough CO2 only to cover the containers. Make sure that the containers are always covered with CO2 (i.e., deactivate the Exchange option before all CO2 has left the chamber). Repeat step 5.5 6x.
- Activate CO2 in one last time and fill the chamber until the containers are covered.
- Activate the Heat option and deactivate the Stirrer option. Close the CO2 cylinder valve.
- Wait until the temperature rises to 35 °C; then, activate the Gas out option.
NOTE: At around 30 °C, the chamber reaches pressures of 70-80 bar, achieving the critical point, where the liquid disappears. - When the chamber pressure reaches 1 bar, all gas content has been removed. Open the chamber and remove the containers.
- Switch off the equipment.
6. Mounting
- Prepare SEM sample holders (i.e., aluminum stubs; Figure 2C.4).
- Wrap the stubs with a piece of aluminum foil, covering only the top, to facilitate stub cleaning after the analysis.
- Identify the stubs by writing the sample code/number on the holder bottom, guaranteeing the identification of what is placed on top.
- Cover the upper part with double-sided carbon tape. Place the stubs into a specimen holder.
- Open the lid of the specimen container, and carefully transfer the dried sample to a glass Petri dish using forceps and spatula.
NOTE: Critical point-dried gardens tend to agglomerate forming highly packed samples that should be carefully set apart as fragments no larger than 1 mm3in size. - Carefully place fragments on the sticky surface of the tape-covered stub. Once the garden fragment touches the tape, it is very difficult to (re)move it, so be careful not to place it in unwanted places or positions. Add up to nine fragments per stub.
- Repeat steps 6.2 and 6.3 for each sample.
7. Sputter-coating with gold
NOTE: Coating the sample is required to prevent its charging. Follow the manufacturer's instructions for adjusting settings such as the operation gas pressure (0.5 × 10-1 mm Hg of gas pressure in this protocol), the sputtering time (220 s), thickness of the gold layer (~120 Å), the current (50 mA), and the voltage supply. Sputtering tends to follow a common workflow though the equipment from different manufacturers may operate slightly differently.
- Open up the hinged target arm and place the stubs on the specimen table.
- Close down the hinged target arm and check whether the splinter shield of the glass vacuum chamber is properly embedded.
- Open the argon cylinder valve and switch on the main power switch.
- Follow the vacuum rising in the equipment display until it reaches the 0.5 × 10-1 mm Hg mark in the display; then, activate Rinsing. Repeat the operation 5x.
- Switch on the water circulation system, activate the option HV On, and open the gold-film lid. Confirm that the plasma color is pinkish. To follow this protocol, set up 220 s of sputtering with 50 mA voltage, which will deposit a gold layer of ~120 Å (12 nm).
- HV On is automatically switched off. Turn off the water circulation system and close the gold-film lid.
- Turn off the main power switch, allowing air to enter the vacuum chamber. For garden and waste samples, repeat steps 7.1-7.7 3x.
NOTE: If the imaging will not occur immediately after sample preparation, store the stubs in a hermetic container filled with a silica layer to avoid rehydrating the samples.
8. Imaging
NOTE: Follow the manufacturer's instructions for adjusting SEM settings, such as objective aperture diameter, operating voltage, alignment of the electron beam system, axial alignment, and stigmators.
- Place the stubs in the sample holder, taking notes on each sample's position.
NOTE: Wear gloves when inserting or removing the sample holder, and keep the holder as clean as possible. - Launch the operation software from the desktop.
- Select the instrument settings. Visualize garden samples with the objective aperture diameter of 30 µm (i.e., in the second stage), operating in high vacuum, detecting signals from secondary electrons (SED), accelerating voltage of 20 kV, working distance of 15 - 20 mm, probe current of 40.0 (in high current mode), and varying the magnification according to the sample.
- Following the navigation instructions, press the Vent icon and wait for the specimen chamber to be vented. A progress bar indicates the vacuum status.
- When atmospheric pressure has been reached, open the specimen chamber and carefully insert the sample holder.
- Gently close the chamber door and press the Evac icon to evacuate the instrument, following the vacuum status through the progress bar. The navigation system will show the moving stage position and provide a holder graph when the moving is completed. Press the Camera icon to record a photo of the holder to get a top view that will help to navigate between samples while imaging.
- Press the Observation icon to turn on the electron gun and wait for the image to form. Use the Manual User Interface (manual control) to manually move the Z-axis to the proper height (based on the sample height and the determined working distance).
NOTE: For prolonging the tungsten filament lifetime, pause the Observation function whenever not actively imaging. - Use either the options shown on the screen or the manual control to change the raster rate and image position. Move the stage to get a thorough view of the sample, employing the RDC function to focus on specific areas. When observing a target (or an interesting) structure, adjust the magnification, focus, brightness, contrast, and stigmation accordingly. Correct stigmation by using the Manual User Interface to move the stage to X and Y directions.
- Adjust the magnification between 100x and 700x to visualize general garden aspects (such as hyphal density, substrate, and colonization patterns).
- Adjust the magnification between 700x and 1,500x to visualize the microbiota spatial patterns.
- Adjust the magnification between 1,500x and 3,000x to observe the microbiota and biofilm physical interactions.
- Adjust the magnification between 3,000x and 4,000x to focus on specific microbial clusters.
- To save an image, use the Freeze function, click on the Photo icon, and set up the file path. As a standard, analyze at least three garden fragments, imaging each one in all magnification ranges mentioned in step 8.8. This results in at least 12 images per sample, though more images may support more detailed descriptions. We suggest that 15-25 images per sample, ranging all the magnifications in step 8.8 tend to provide fine details for sample description.
NOTE: Rastering and saving an image takes a few seconds during which any vibration on the SEM floating table should be avoided. - After finishing imaging, press the Vent option to vent the chamber, following the vacuum status through the progress bar. Carefully remove the holder, gently close the chamber door, and press Evac to evacuate the chamber.
- As soon as the evacuation is completed, exit the operation software.
- Adjust the brightness and contrast to improve visualization using an image editor.
Results
Here, we presented a simplified protocol to visualize the components of attine garden and waste samples, such as fungal hyphae, substrate, microbiota, and biofilms. SEM has enhanced our understanding of how the garden and waste scaffold the microbiota structural patterns (Figure 3). In attine gardens, fungal hyphae are branch-like structures covering portions of the substrate surface. Since fungal hyphae tend to be very sensitive to dehydration and rupture, the user may be guided by the hyph...
Discussion
SEM uses an electron beam to scan the sample, generating an enlarged image of it such that one can visualize three-dimensional microstructures in high resolution. As SEM operates under high vacuum, the removal of up to/more than 99% of water from samples is required. Inside the SEM vacuum chamber, partially hydrated samples may dehydrate and collapse, besides scattering electrons. For high-resolution imaging in SEM, sample preparation should include procedures for removing water while keeping the changes in volume and mo...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for providing financial support (Grant #2019/03746-0). MOB thanks for PhD scholarship received from FAPESP (process 2021/08013-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. AR also thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a research fellowship (#305269/2018). The authors would like to thank Marcia Regina de Moura Aouada and Antonio Teruyoshi Yabuki for helping with pilot tests for sample preparation, to Renato Barbosa Salaroli for technical assistance, and to Enzo Sorrentino for helping in photo shooting. This study was carried out under the access genetic heritage authorization # SISGen AA39A6D.
Materials
| Name | Company | Catalog Number | Comments |
| 2 mL tube | Axygen | MCT-200-C-BRA | To fix and dehydrate samples |
| Calcium chloride anhydrous | Merck | C4901 | CaCl2 anhydrous to prepare Karnovsky’s fixative |
| Critical point dryer | Leica | EM CPD 300 | For critical point drying |
| Double Sided Carbon Conductive Tape, 12 mm (W) X 5 M (L) | Electron Microscopy Sciences | 77819-12 | For mounting samples |
| Entomological forceps | No specific supplier | To manipulate garden samples | |
| Ethyl alcohol (=ethanol), pure (≥99.5%) | Sigma-Aldrich | 459836 | For dehydration |
| Forceps | No specific supplier | To manipulate garden samples | |
| Glass beaker | No specific supplier | For dehydration | |
| Glass Petri dish | No specific supplier | To manipulate garden samples | |
| Glass pipette | No specific supplier | To fix and dehydrate samples | |
| Glutaraldehyde (Aqueous Glutaraldehyde EM Grade 25%) | Electron Microscopy Sciences | 16220 | To prepare Karnovsky’s fixative |
| Gold target | Ted Pella, Inc. | 8071 | To sputter coat with gold |
| Hydrochloric acid | Sigma-Aldrich | 320331 | For adjusting solutions pH |
| Image editor | Photoshop | any version | To adjust images |
| Paraformaldehyde (Paraformaldehyde 20% Aqueous Solution EM Grade) | Electron Microscopy Sciences | 15713 | To prepare Karnovsky’s fixative |
| Propilene recipient | No specific supplier | For maintaining alive ant colonies | |
| Scanning Electron Microscope | JEOL | IT300 SEM | For sample imaging |
| Sodium cacodylate trihydrate | Sigma-Aldrich | C0250 | For preparing sodium cacodylate buffer |
| Spatula | No specific supplier | To manipulate garden samples | |
| Specimen containers with 15 mm dia. x 10 mm high | Ted Pella, Inc. | 4591 | For critical point drying |
| Sputter coater | Baltec | SCD 050 | To coat with gold |
| Stub (Aluminium mount, flat end pin) 12.7 mm x 8 mm | Electron Microscopy Sciences | 75520 | For mounting samples |
References
- Turner, M. G. Landscape ecology: the effect of pattern on process. Annu Rev Ecol Evol Syst. 20 (1), 171-197 (1989).
- Rietkerk, M., Van de Koppel, J. Regular pattern formation in real ecosystems. Trends Ecol Evol. 23 (3), 169-175 (2008).
- Schmitz, O. J. Spatial dynamics and ecosystem functioning. PLOS Biol. 8 (5), e1000378 (2010).
- Pringle, R. M., Doak, D. F., Brody, A. K., Jocqué, R., Palmer, T. M. Spatial pattern enhances ecosystem functioning in an African savanna. PLOS Biol. 8 (5), e1000377 (2010).
- Wimpenny, J. W. Spatial order in microbial ecosystems. Biol Rev. 56 (3), 295-342 (1981).
- Martiny, J. B. H., et al. Microbial biogeography: putting microorganisms on the map. Nat Rev. Microbiol. 4 (2), 102-112 (2006).
- McCallum, G., Tropini, C. The gut microbiota and its biogeography. Nat Rev Microbiol. 22 (2), 105-118 (2024).
- Lamont, R. J., Hajishengallis, G., Koo, H. Social networking at the microbiome-host interface. Infec Immun. 91 (9), e00124-e00223 (2023).
- Welch, J. L. M., Rossetti, B. J., Rieken, C. W., Dewhirst, F. E., Borisy, G. G. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci USA. 113 (6), E791-E800 (2016).
- Kim, D., et al. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc Natl Acad Sci USA. 117 (22), 12375-12386 (2020).
- Swidsinski, A., Weber, J., Loening-Baucke, V., Hale, L. P., Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 43 (7), 3380-3389 (2005).
- Ibberson, C. B., Barraza, J. P., Holmes, A. L., Cao, P., Whiteley, M. Precise spatial structure impacts antimicrobial susceptibility of S. aureus in polymicrobial wound infections. Proc Natl Acad Sci USA. 119 (51), e2212340119 (2022).
- Azimi, S., Lewin, G. R., Whiteley, M. The biogeography of infection revisited. Nat Rev Microbiol. 20 (10), 579-592 (2022).
- Dejea, C. M., et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA. 111 (51), 18321-18326 (2014).
- Hanson, C. A., Fuhrman, J. A., Horner-Devine, M. C., Martiny, J. B. Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol. 10 (7), 497-506 (2012).
- Adade, E. E., Al Lakhen, K., Lemus, A. A., Valm, A. M. Recent progress in analyzing the spatial structure of the human microbiome: Distinguishing biogeography and architecture in the oral and gut communities. Curr Opin Endocr. 18, 275-283 (2021).
- Mony, C., Bohannan, B. J., Leibold, M. A., Peay, K., Vandenkoornhuyse, P. Microbial landscape ecology: Highlights on the invisible corridors. Front Ecol Evol. 9, 753213 (2021).
- Hölldobler, B., Wilson, E. O. . The Ants. , (1990).
- Chapela, I. H., Rehner, S. A., Schultz, T. R., Mueller, U. G. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science. 266 (5191), 1691-1694 (1994).
- Mueller, U. G., Rehner, S. A., Schultz, T. R. The evolution of agriculture in ants. Science. 281 (5385), 2034-2038 (1998).
- Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L., Schultz, T. R. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 36, 563-595 (2005).
- Dentinger, B. T., Lodge, D. J., Munkacsi, A. B., Desjardin, D. E., McLaughlin, D. J. Phylogenetic placement of an unusual coral mushroom challenges the classic hypothesis of strict coevolution in the Apterostigma pilosum group ant-fungus mutualism. Evolution. 63 (8), 2172-2178 (2009).
- Schultz, T. R., Brady, S. G. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA. 105, 5435-5440 (2008).
- de Fine Licht, H. H., Boomsma, J. J. Forage collection, substrate preparation, and diet composition in fungus-growing ants. Ecol Entomol. 35 (3), 259-269 (2010).
- Martin, M. M. The biochemical basis of the fungus-attine ant symbiosis: A complex symbiosis is based upon integration of the carbon and nitrogen metabolisms of the two organisms. Science. 169 (3940), 16-20 (1970).
- Grell, M. N., et al. The fungal symbiont of Acromyrmex leaf-cutting ants expresses the full spectrum of genes to degrade cellulose and other plant cell wall polysaccharides. BMC Genomics. 14, 928 (2013).
- Lange, L., Grell, M. N. The prominent role of fungi and fungal enzymes in the ant-fungus biomass conversion symbiosis. Appl Microbiol Biotechnol. 98, 4839-4851 (2014).
- Huang, E. L., et al. The fungus gardens of leaf-cutter ants undergo a distinct physiological transition during biomass degradation. Environ Microbiol Rep. 6 (4), 389-395 (2014).
- Craven, S. E., Dix, M. W., Michaels, G. E. Attine fungus gardens contain yeasts. Science. 169 (3941), 184-186 (1970).
- De Fine Licht, H. H., Boomsma, J. J., Tunlid, A. Symbiotic adaptations in the fungal cultivar of leaf-cutting ants. Nat Commun. 5 (1), 5675 (2014).
- Aylward, F. O., et al. Enrichment and broad representation of plant biomass-degrading enzymes in the specialized hyphal swellings of Leucoagaricus gongylophorus, the fungal symbiont of leaf-cutter ants. PLoS One. 10 (8), e0134752 (2015).
- Moreira-Soto, R. D., Sanchez, E., Currie, C. R., Pinto-Tomás, A. A. Ultrastructural and microbial analyses of cellulose degradation in leaf-cutter ant colonies. Microbiology. 163 (11), 1578-1589 (2017).
- Erthal Jr, M., Silva, C. P., Cooper, R. M., Samuels, R. I. Hydrolytic enzymes of leaf-cutting ant fungi. Comp Biochem. 152 (1), 54-59 (2009).
- North, R. D., Jackson, C. W., Howse, P. E. Communication between the fungus garden and workers of the leaf-cutting ant, Atta sexdens rubropilosa, regarding choice of substrate for the fungus. Physiol Entomol. 24 (2), 127-133 (1999).
- Herz, H., Hölldobler, B., Roces, F. Delayed rejection in a leaf-cutting ant after foraging on plants unsuitable for the symbiotic fungus. Behav Ecol. 19 (3), 575-582 (2008).
- Schiøtt, M., De Fine Licht, H. H., Lange, L., Boomsma, J. J. Towards a molecular understanding of symbiont function: identification of a fungal gene for the degradation of xylan in the fungus gardens of leaf-cutting ants. BMC Microbiol. 8, 40 (2008).
- Bot, A. N., Currie, C. R., Hart, A. G., Boomsma, J. J. Waste management in leaf-cutting ants. Ethol Ecol Evol. 13 (3), 225-237 (2001).
- Scott, J. J., et al. Microbial community structure of leaf-cutter ant fungus gardens and refuse dumps. PLOS One. 5 (3), e9922 (2010).
- Lewin, G. R., et al. Cellulose-enriched microbial communities from leaf-cutter ant (Atta colombica) refuse dumps vary in taxonomic composition and degradation ability. PLOS One. 11 (3), e0151840 (2016).
- Suen, G., et al. An insect herbivore microbiome with high plant biomass-degrading capacity. PLOS Genet. 6 (9), e1001129 (2010).
- Aylward, F. O., et al. Convergent bacterial microbiotas in the fungal agricultural systems of insects. MBio. 5 (6), e02077 (2014).
- Barcoto, M. O., et al. Fungus-growing insects host a distinctive microbiota apparently adapted to the fungiculture environment. Sci Rep. 10 (1), 12384 (2020).
- Francoeur, C. B., et al. Bacteria contribute to plant secondary compound degradation in a generalist herbivore system. mBio. 11, e02146-e02220 (2020).
- Martiarena, M. J. S., Deveau, A., Montoya, Q. V., Flórez, L. V., Rodrigues, A. The hyphosphere of leaf-cutting ant cultivars is enriched with helper bacteria. Microb Ecol. 86 (3), 1773-1788 (2023).
- Leal-Dutra, C. A., et al. Evidence that the domesticated fungus Leucoagaricus gongylophorus recycles its cytoplasmic contents as nutritional rewards to feed its leafcutter ant farmers. IMA Fungus. 14 (1), 19 (2023).
- Sosa-Calvo, J., Jesovnik, A., Okonski, E., Schultz, T. R. Locating, collecting, and maintaining colonies of fungus-farming ants (Hymenoptera: Myrmicinae: Attini). Sociobiology. 62 (2), 300-320 (2015).
- Karnovsky, M. J. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol. 27, 137-138 (1965).
- Eltoum, I., Fredenburgh, J., Myers, R. B., Grizzle, W. E. Introduction to the theory and practice of fixation of tissues. J Histotechnol. 24 (3), 173-190 (2001).
- Gusnard, D., Kirschner, R. H. Cell and organelle shrinkage during preparation for scanning electron microscopy: effects of fixation, dehydration and critical point drying. J Microsc. 110 (1), 51-57 (1977).
- Surman, S. B., et al. Comparison of microscope techniques for the examination of biofilms. J Microbiol Methods. 25 (1), 57-70 (1996).
- Augustin, J. O., et al. Yet more "weeds" in the garden: fungal novelties from nests of leaf-cutting ants. PLOS One. 8 (12), e82265 (2013).
- Montoya, Q. V., Martiarena, M. J. S., Polezel, D. A., Rodrigues, A. More pieces to a huge puzzle: Two new Escovopsis species from fungus gardens of attine ants. MycoKeys. 46, 97 (2019).
- Varanda-Haifig, S. S., et al. Nature of the interactions between hypocrealean fungi and the mutualistic fungus of leaf-cutter ants. Antonie van Leeuwenhoek. 110, 593-605 (2017).
- Custodio, B. C., Rodrigues, A. Escovopsis kreiselii specialization to its native hosts in the fungiculture of the lower attine ant Mycetophylax morschi. Antonie van Leeuwenhoek. 112, 305-317 (2019).
- Schröttner, H., Schmied, M., Scherer, S. Comparison of 3D surface reconstruction data from certified depth standards obtained by SEM and an Infinite Focus Measurement Machine (IFM). Microchim Acta. 155, 279-284 (2006).
- Zhou, W., Apkarian, R., Wang, Z. L., Joy, D., Zhou, W., Wang, Z. L. Fundamentals of scanning electron microscopy (SEM). Scanning microscopy for nanotechnology: Techniques and applications. , 1-40 (2007).
- Kannan, M., Raja, K., Subramanian, K. S., Kannan, M. Scanning electron microscopy: Principle, components and applications. A Textbook on Fundamentals and Applications of Nanotechnology. , 81-92 (2018).
- Ruffolo, J. J. Critical point drying of protozoan cells and other biological specimens for scanning electron microscopy: apparatus and methods of specimen preparation. Trans Am Microsc. 93 (1), 124-131 (1974).
- Echlin, P. Recent advances in specimen coating techniques for electron microscopy. Scanning Electron Microscopy 1981/1. , 79-90 (1981).
- Ris, H. The cytoplasmic filament system in critical point-dried whole mounts and plastic-embedded sections. J Cell Biol. 100 (5), 1474-1487 (1985).
- Bray, D. F., Bagu, J., Koegler, P. Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens. Microsc Res Tech. 26 (6), 489-495 (1993).
- Bergmans, L., Moisiadis, P., Van Meerbeek, B., Quirynen, M., Lambrechts, P. Microscopic observation of bacteria: review highlighting the use of environmental SEM. Int Endod J. 38 (11), 775-788 (2005).
- Kirk, S. E., Skepper, J. N., Donald, A. M. Application of environmental scanning electron microscopy to determine biological surface structure. J Microsc. 233 (2), 205-224 (2009).
- Kemmenoe, B. H., Bullock, G. R. Structure analysis of sputter-coated and ion-beam sputter-coated films: a comparative study. J Microsc. 132 (2), 153-163 (1983).
- Kinden, D. A., Brown, M. F. Technique for scanning electron microscopy of fungal structures within plant cells. Phytopathology. 65, 74-76 (1975).
- Masaphy, S., Levanon, D., Tchelet, R., Henis, Y. Scanning electron microscope studies of interactions between Agaricus bisporus (Lang) Sing hyphae and bacteria in casing soil. Appl Environ Microbiol. 53 (5), 1132-1137 (1987).
- Massicotte, H. B., Melville, L. H., Peterson, R. L. Scanning electron microscopy of ectomycorrhizae potential and limitations. Scanning Microsc. 1 (3), 58 (1987).
- Visen, A., Singh, P. N., Chakraborty, B., Singh, A., Bisht, T. S. Scanning electron microscopy indicates Pseudomonad strains facilitate AMF mycorrhization in litchi (Litchi chinensis Sonn.) air-layers and improving survivability, growth and leaf nutrient status. Curr Res Microb Sci. 2, 100063 (2021).
- Fleeman, R. M., Mikesh, M., Davies, B. W. Investigating Klebsiella pneumoniae biofilm preservation for scanning electron microscopy. Access Microbiol. 5 (2), 000470-000473 (2023).
- Wells, M., Mikesh, M., Gordon, V. Structure-preserving fixation allows scanning electron microscopy to reveal biofilm microstructure and interactions with immune cells. J Microsc. 293 (1), 59-68 (2024).
- Nadell, C. D., Drescher, K., Foster, K. R. Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol. 14 (9), 589-600 (2016).
- Madsen, J. S., et al. Coexistence facilitates interspecific biofilm formation in complex microbial communities. Environ Microbiol. 18 (8), 2565-2574 (2016).
- Madsen, J. S., Sørensen, S. J., Burmølle, M. Bacterial social interactions and the emergence of community-intrinsic properties. Curr Opin Microbiol. 42, 104-109 (2018).
- Flemming, H. C., Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol. 17 (4), 247-260 (2019).
- Bringhurst, B., Allert, M., Greenwold, M., Kellner, K., Seal, J. N. Environments and hosts structure the bacterial microbiomes of fungus-gardening ants and their symbiotic fungus gardens. Microb Ecol. 86 (2), 1374-1392 (2023).
- Bringhurst, B., Greenwold, M., Kellner, K., Seal, J. N. Symbiosis, dysbiosis and the impact of horizontal exchange on bacterial microbiomes in higher fungus-gardening ants. Sci Rep. 14 (1), 3231 (2024).
- Vargas, S., et al. Body-plan reorganization in a sponge correlates with microbiome change. Mol Biol Evol. 40 (6), msad138 (2023).
- Apprill, A., et al. Humpback whale populations share a core skin bacterial community: towards a health index for marine mammals. PLOS One. 9 (3), e90785 (2014).
- Fraune, S., et al. Bacteria-bacteria interactions within the microbiota of the ancestral metazoan Hydra contribute to fungal resistance. ISME J. 9 (7), 1543-1556 (2015).
- Gowen, R., Gamal, A., Di Martino, L., McCormick, T. S., Ghannoum, M. A. Modulating the microbiome for Crohn's disease treatment. Gastroenterology. 164 (5), 828-840 (2023).
- Moya, P., et al. Molecular phylogeny and ultrastructure of the lichen microalga Asterochloris mediterranea sp. nov. from Mediterranean and Canary Islands ecosystems. Int J Syst Evol Microbiol. 65 (6), 1838-1854 (2015).
- Zettler, E. R., Mincer, T. J., Amaral-Zettler, L. A. Life in the "plastisphere": microbial communities on plastic marine debris. Environ Sci Technol. 47 (13), 7137-7146 (2013).
- Porter, K. R., Kallman, F. The properties and effects of osmium tetroxide as a tissue fixative with special reference to its use for electron microscopy. Exp Cell Res. 4 (1), 127-141 (1953).
- Ligon, J. J., Abraham, J. L., Boyd, A. S. Traumatic osmium tetroxide inoculation. J Am Acad Dermatol. 45 (6), 949-952 (2001).
- Friedova, N., et al. Osmium absorption after osmium tetroxide skin and eye exposure. Basic Clin Pharmacol Toxicol. 127 (5), 429-433 (2020).
- Fratesi, S. E., Lynch, F. L., Kirkland, B. L., Brown, L. R. Effects of SEM preparation techniques on the appearance of bacteria and biofilms in the Carter Sandstone. J Sediment. 74 (6), 858-867 (2004).
- Dassanayake, R. P., et al. Identification of a reliable fixative solution to preserve the complex architecture of bacterial biofilms for scanning electron microscopy evaluation. PLOS One. 15 (5), e0233973 (2020).
- Desiro, A., et al. Detection of a novel intracellular microbiome hosted in arbuscular mycorrhizal fungi. ISME J. 8 (2), 257-270 (2014).
- Morales, D. P., et al. Advances and challenges in fluorescence in situ hybridization for visualizing fungal endobacteria. Front Microbiol. 13, 892227 (2022).
- Shi, H., et al. Highly multiplexed spatial mapping of microbial communities. Nature. 588 (7839), 676-681 (2020).
- Valm, A. M., Welch, J. L. M., Borisy, G. G. CLASI-FISH: principles of combinatorial labeling and spectral imaging. Syst Appl Microbiol. 35 (8), 496-502 (2012).
- Fernandez-Brime, S., Muggia, L., Maier, S., Grube, M., Wedin, M. Bacterial communities in an optional lichen symbiosis are determined by substrate, not algal photobionts. FEMS Microbiol Ecol. 95 (3), fiz012 (2019).
- Schluter, S., Eickhorst, T., Mueller, C. W. Correlative imaging reveals holistic view of soil microenvironments. Environ Sci Technol. 53 (2), 829-837 (2018).
- Barcoto, M. O., Rodrigues, A. Lessons from insect fungiculture: from microbial ecology to plastics degradation. Front Microbiol. 13, 812143 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved