A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively high concentration).
Concentrations may be quantitatively assessed using a wide variety of measurement units, each convenient for particular applications. Molarity (M) is a useful concentration unit for many applications in chemistry. Molarity is defined as the amount of solute in number of moles divided by the volume of the solution in liters:
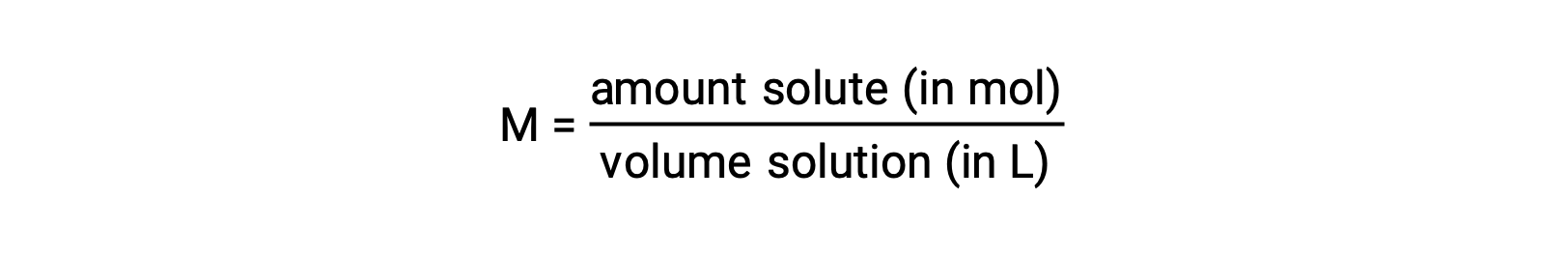
Because solution volumes vary with temperature, molar concentrations will likewise vary. When expressed as molarity, the concentration of a solution with identical numbers of solute and solvent species will be different at different temperatures due to the contraction/expansion of the solution. More appropriate for calculations involving many colligative properties are mole-based concentration units whose values are not dependent on temperature. Two such units are mole fraction (introduced in the previous chapter on gases) and molality.
The mole fraction, χA, of a component is the ratio of its molar amount to the total number of moles of all solution components:
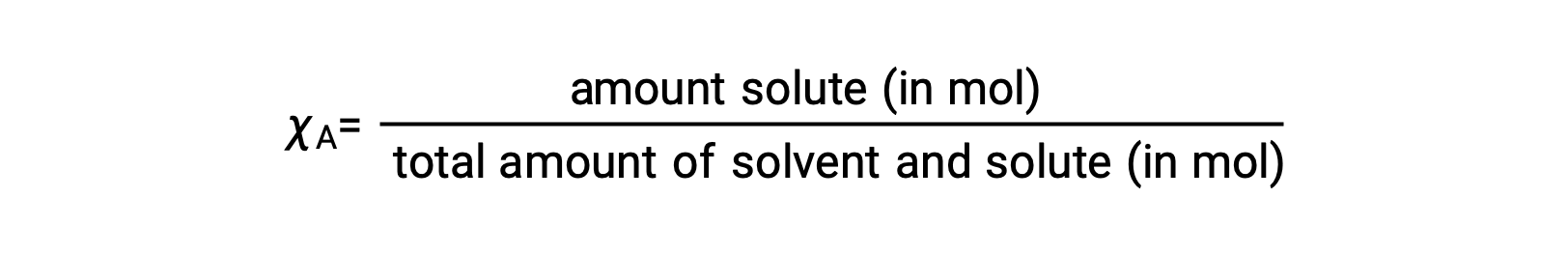
By this definition, the sum of mole fractions for all solution components (the solvent and all solutes) is equal to one.
Molality is a concentration unit defined as the ratio of the numbers of moles of solute to the mass of the solvent in kilograms:
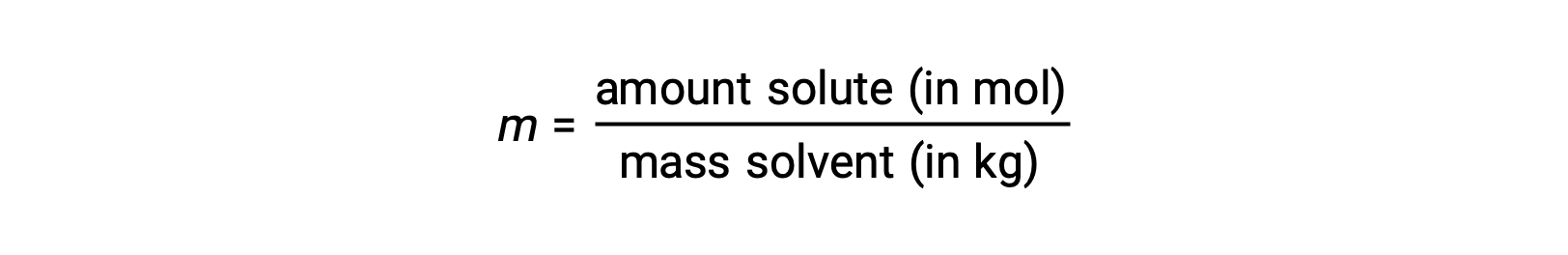
Since these units are computed using only masses and molar amounts, they do not vary with temperature and, thus, are better suited for applications requiring temperature-independent concentrations.
This text is adapted from Openstax, Chemistry 2e, Section 11.4: Colligative Properties.
Do Capítulo 12:

Now Playing
12.7 : Expressing Solution Concentration
Solutions and Colloids
57.6K Visualizações

12.1 : Solution Formation
Solutions and Colloids
30.4K Visualizações

12.2 : Intermolecular Forces in Solutions
Solutions and Colloids
32.1K Visualizações

12.3 : Enthalpy of Solution
Solutions and Colloids
24.2K Visualizações

12.4 : Aqueous Solutions and Heats of Hydration
Solutions and Colloids
13.9K Visualizações

12.5 : Solution Equilibrium and Saturation
Solutions and Colloids
17.9K Visualizações

12.6 : Physical Properties Affecting Solubility
Solutions and Colloids
21.9K Visualizações

12.8 : Vapor Pressure Lowering
Solutions and Colloids
25.0K Visualizações

12.9 : Ideal Solutions
Solutions and Colloids
18.5K Visualizações

12.10 : Freezing Point Depression and Boiling Point Elevation
Solutions and Colloids
33.2K Visualizações

12.11 : Osmosis and Osmotic Pressure of Solutions
Solutions and Colloids
38.2K Visualizações

12.12 : Electrolytes: van't Hoff Factor
Solutions and Colloids
32.1K Visualizações

12.13 : Colloids
Solutions and Colloids
17.1K Visualizações
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados