Organocatalysis
Source: Vy M. Dong and Faben Cruz, Department of Chemistry, University of California, Irvine, CA
This experiment will demonstrate the concept of organocatalysis by illustrating the proper setup of a reaction that utilizes enamine catalysis. Organocatalysis is a form of catalysis that uses substoichiometric amounts of small organic molecules to accelerate reactions. This type of catalysis is complementary to other forms of catalysis such as transition metal or biocatalysis. Transition metal catalysis involves transition metals as catalysts and biocatalysis uses enzymes as catalysts. Some advantages of organocatalysis include the low toxicity and cost of the organocatalysts in comparison to many metal catalysts. In addition, most organocatalysts are not sensitive to air and moisture, unlike metal catalysts. In contrast to enzymes found in living organisms, the small molecules that act as organocatalysts are typically easy to access. Furthermore, organocatalysis offers complementary and new reactivity not observed with other forms of catalysis.
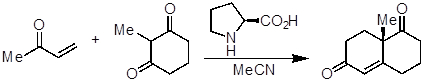
- Add (S)-proline (40 mg, 0.35 mmol, 0.35 equivalents), acetonitrile (MeCN, 5 mL), and the diketone (126 mg, 1 mmol, 1 equivalent) to a round-bottom flask (~ 20 mL) equipped with a magnetic stir bar.
- Stir the reaction mixture at 35 °C for 30 min.
- Add 3-buten-2-one (105 mg, 1.5 mmol, 1.5 equivalents) dropwise at 35 °C and stir at the same temperature for 1 week.
- Cool the reaction to room temperat
This experiment has demonstrated how to set up an enamine catalyzed reaction. Compared to other forms of catalysis, organocatalysis is a relatively young field of research, but in recent years the field of organocatalysis has experienced dramatic growth. The increased interest in organocatalysis has also given rise to research that makes use of more than one type of catalysis to achieve new types of reactivity. For example, there has been increased reports of using organocatalysis in conjunction with transition metal cat
Перейти к...
Видео из этой коллекции:

Now Playing
Organocatalysis
Organic Chemistry II
16.6K Просмотры

Cleaning Glassware
Organic Chemistry II
123.1K Просмотры

Nucleophilic Substitution
Organic Chemistry II
99.2K Просмотры

Reducing Agents
Organic Chemistry II
42.7K Просмотры

Grignard Reaction
Organic Chemistry II
148.7K Просмотры

n-Butyllithium Titration
Organic Chemistry II
47.6K Просмотры

Dean-Stark Trap
Organic Chemistry II
99.7K Просмотры

Ozonolysis of Alkenes
Organic Chemistry II
66.8K Просмотры

Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.2K Просмотры

Solid Phase Synthesis
Organic Chemistry II
40.8K Просмотры

Hydrogenation
Organic Chemistry II
49.1K Просмотры

Polymerization
Organic Chemistry II
93.7K Просмотры

Melting Point
Organic Chemistry II
149.6K Просмотры

Infrared Spectroscopy
Organic Chemistry II
213.8K Просмотры

Polarimeter
Organic Chemistry II
99.8K Просмотры
Авторские права © 2025 MyJoVE Corporation. Все права защищены