A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Z-Scores for Assessing Ovarian Reserve in Young Patients Undergoing Fertility Preservation
In This Article
Summary
The present protocol describes a method for assessing ovarian reserve in patients under 25 years old who require fertility preservation through ovarian tissue cryopreservation. This method involves: (1) histological assessment of ovarian reserve in cortical samples, (2) comparison to a reference dataset, and (3) calculation of Z-scores.
Abstract
Women are born with a non-renewable pool of ovarian follicles, referred to as the ovarian reserve. This reserve consists of primordial follicles in the ovaries and can be affected by many factors, such as genetic and endocrine disorders, medical interventions, and endocrine disruptors. Fertility preservation is recommended when gonadotoxic treatments are necessary. The preferred options for women are oocyte and embryo cryopreservation. However, in very young, sexually immature patients, ovarian tissue cryopreservation is the only option. Knowing the follicular density of cryopreserved tissue samples is essential in fertility counseling for young patients. This protocol demonstrates the use of Z-scores for cortical follicle density as a tool to evaluate the quality of ovarian tissue in girls and young women aged 25 years and below who are undergoing fertility preservation. Follicle density in patient samples is compared to age-normalized reference standards, developed by Hassan et al. to estimate possible deviations from the standard. The follicle density is measured through histological quantification. For this, a small piece of ovarian cortical tissue (~2 mm x 2 mm x 2 mm) is fixed in either Bouin's or formaldehyde solution, embedded in paraffin, sectioned at 4 µm thickness, stained with hematoxylin and eosin, and digitized using a slide scanner. Follicular stages present in the cortex range from primordial to primary follicles. The cortical area was 1 mm from the surface epithelium on histological sections. Follicle density is calculated using a modified formula presented by Schmidt et al., and the Z-score is determined using the reference mean and standard deviation. The Z-score indicates how much the measured value deviates from the reference mean, determining reduced (<-1.7 Z-score) ovarian reserve. With this method, follicle densities can be used as a valuable measure of ovarian reserve for young patients requiring fertility preservation.
Introduction
The ovary is a dynamic, endocrinologically active organ, consisting of an outer cortex and an inner medulla. Heterogeneously distributed within the ovaries are follicles, which contain the gonadal oocyte surrounded by stromal granulosa cells1. All follicles are already formed during fetal development, peaking at around 20 weeks of gestational age, followed by an exponential decline. At birth, 1-2 million primordial follicles remain within the cortex, and only a few hundred thousand follicles survive until puberty2. The non-growing follicle pool, also referred to as the ovarian reserve, influences a woman's fertility, among other factors. The ovarian reserve resides within the cortex and moves into the medulla as the follicles grow and mature. Some researchers recognize the ovarian reserve as being comprised of primordial follicles only, while others include all unilaminar follicles (i.e., primordial, intermediary, and primary)3,4,5,6. During a woman's lifetime, only 300-400 follicles mature and survive to eventually undergo ovulation. Menopause commences when the follicle number drops to around a thousand, which typically occurs at around 50 years of age2,7. There is currently an ongoing debate regarding the presence of oogonial stem cells (OSCs) in adult human ovaries that can form oocytes after xenotransplantation. Some studies suggest isolating cells that express a gene profile of primitive germ cells and differentiate into oocytes8,9, while others indicate that the alleged OSCs are perivascular cells of the blood vessels10,11,12.
Premature ovarian insufficiency (POI) is defined by the discontinuation of the menstrual cycle with raised FSH levels before the age of 40 years13. The causes of POI are multifactorial and can occur idiopathically; however, it often arises as a result of medical conditions14. For example, certain congenital genetic conditions are associated with POI due to a reduced ovarian reserve already at birth, such as Turner's syndrome15. POI can also occur as a side effect of gonadotoxic treatments, which is particularly problematic in very young patients as it may lead to an inability to undergo normal puberty and drastically impair the ovarian reserve. Depending on the risk of POI and the patient's age, several fertility preservation options are available, such as cryopreservation of mature oocytes, cryopreservation of embryos after in vitro fertilization, and ovarian tissue cryopreservation (OTC). For pre-pubertal patients, OTC is the only feasible option due to their sexual immaturity16,17. In the United States, fertility preservation through OTC has recently been considered an acceptable clinical routine18. However, OTC is still considered experimental in many European countries, and ethical approval is required, particularly for auto-transplantation19,20. Therefore, inclusion criteria for OTC protocols must be carefully considered, and a risk-benefit analysis should be performed for each patient. Not all children receiving gonadotoxic therapies are eligible for fertility preservation. In Sweden, only patients with high or very high risk are recommended to undergo fertility preservation in accordance with the guidelines on fertility preservation of the Nordic Society of Paediatric Haematology and Oncology21. This group includes (1) pre- and post-pubertal children requiring stem cell transplantation or receiving radiation therapy with the ovary in the field of range, and (2) post-pubertal children with high cumulative doses of alkylating chemotherapy. For girls before menarche, inclusion criteria for very high risk of infertility include those treated with any of the following: radiation dose >10 Gy to the ovary, or allogeneic or autologous hematopoietic stem cell therapy (HSCT). Exclusion criteria include those who need additional blood products for other anticoagulant preparations before surgery or have a neutrophil count <1. For girls/women after menarche, inclusion criteria for high or very high risk of infertility include those treated with any of the following: radiation dose >10 Gy to the ovary, or allogeneic HSCT, cyclophosphamide >9 g/m², ifosfamide >60 g/m², procarbazine, or nitrosourea compounds (BCNU/CCNU) >360 mg/m². Exclusion criteria include those with an increased risk of bleeding, increased risk of infection, increased risk of surgical complications, or increased risk of pain problems as assessed by the physician in charge.
For OTC, ovarian tissue is collected during abdominal surgeries by laparotomy or laparoscopy through unilateral oophorectomy or biopsy. The medulla is removed from the collected ovarian tissue, and the remaining cortex is then sectioned into smaller fragments for cryopreservation through controlled-rate freezing or vitrification for later use in fertility restoration22. The samples are then stored in liquid nitrogen until the patient recovers from her treatment and wishes to start a family. Depending on the type of cancer and in the absence of contraindications to ovarian tissue transplantation, the tissue is then thawed and autografted to restore hormonal and ovarian function. OTC has been proven successful, with a 50% pregnancy rate and more than 200 live births reported after grafting of cryopreserved ovarian tissue collected during adulthood23,24,25. Reported live births after auto-transplantation of ovarian tissue collected during childhood are few, and none have been reported after auto-transplantation of undoubtedly pre-pubertal ovarian tissue26,27,28,29,30. Tissue quality is a prerequisite for successful fertility restoration31. Therefore, it is essential to study the quality of the collected tissue samples.
Currently, a way of estimating the quality of the ovarian tissue is through histological sections and manual counting of the follicles. However, assessing the normality of follicle density is difficult due to the natural decline of the reserve with increasing age and the heterogeneous distribution of follicles. Additionally, comparison to reference values is challenging as they are not standardized for biopsy size and age. Other assessment options, such as AMH serum levels or staining of digested tissue with calcein or neutral red, are either not well established or come with their own limitations. Wallace and Kelsey previously published a model of normal ovarian reserve over age by collecting data on follicle numbers from whole ovaries of patients with no known ovarian pathologies2. Furthermore, as biopsies are not representative of the whole ovary, Schmidt et al. developed a method for estimating the total number of follicles based on cortical biopsies32. Using a modified version of this method and the model of normal ovarian reserve, a reference dataset for follicle density of women aged 0-25 years was established33.
This article describes the strategy for assessing the normality of follicle density based on small ovarian samples by (1) direct quantification of the follicles using histology and (2) comparison of the results to age-normalized standard references via Z-scores. The use of Z-scores describes the relationship of an ovarian biopy's follicle density to an age- and size-standardized reference group mean. This will be of great value for physicians in counseling their patients for auto-transplantation and further treatment after fertility preservation.
Protocol
All of the procedures conducted were approved by the Swedish Ethical Review Authority (Sveafertil patients, Dnr: 2019-03802) and by the Ethics Committee of Helsinki University Hospital (HCH patients, Dnr 340/13/03/03/2015). Written and oral information about the study were provided by the research nurses, and informed consent was obtained in accordance with the Declaration of Helsinki. For patients under 18 years old, informed consent was obtained from the legal guardians. All samples were pseudonymized and processed according to the European General Data Protection Regulation (GDPR). The details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Ovarian tissue collection and processing
NOTE: Identify all eligible patients for fertility preservation when there is an indication for a high or very high risk of infertility following local guidelines (e.g., Nordic, European, or international specific guidelines). In Europe, recruit underage patients only as part of fertility preservation research programs. Determine the risk of infertility using your country's guidelines for fertility preservation.
- Perform the surgical procedure under general anesthesia. Administer intravenous propofol (2 mg/kg), sufentanil (0.5 microgram/kg), and rocuronium bromide (0.6 mg/kg) for the induction of anesthesia.
- Intubate and ventilate the patient with a tidal volume of 6-8 mL/kg and positive expiratory pressure of 6-8 cm of H2O. Deliver sevoflurane 1% to 2% via the endotracheal tube to maintain inhalation anesthesia.
- Maintain neuromuscular blockade with rocuronium bromide (0.15 mg/kg) during the surgical procedure to provide adequate muscle relaxation and to allow laparoscopy at lower intra-abdominal insufflation pressures.
- Use bispectral index to guide anesthetic depth to facilitate rapid awakening with a reduced anesthetic dose. Antagonize residual neuromuscular block with sugammadex (2-4 mg/kg).
- Using a three-port technique, insert a 10 mm trocar into the peritoneal cavity at the umbilicus to introduce an optical lens with a camera attached, and two 5 mm trocars at the left and right iliac regions (2 cm above and 2 cm medial to the respective anterior superior iliac spine) under direct vision for the surgical instrumentation and tissue retrieval. Inspect the pelvic organs, including both ovaries, to confirm normality.
- Identify the ovary that is easiest to access, then grasp it. Cut a volume that is approximately one-third of the ovary (or as indicated in your ethical permit), then use diathermy, if needed, to secure hemostasis.
NOTE: Perform the biopsy only if there are no contraindications, such as risk factors for excessive intra- or post-operative hemorrhage or increased risk of infection. Specify clearly and follow the country's inclusion and exclusion criteria. The entire ovary can be taken if the risk of infertility is very high and/or the risk of cutting a part only is considered too high (e.g., due to bleeding). Avoid using diathermy before collecting the sample because it can damage the tissue.
- Identify the ovary that is easiest to access, then grasp it. Cut a volume that is approximately one-third of the ovary (or as indicated in your ethical permit), then use diathermy, if needed, to secure hemostasis.
- While in the operating theater, place the tissue in a tube containing 5 mL of 1x Dulbecco's Phosphate Buffer Saline (PBS) with CaCl2, MgCl2, 1 g/L D-glucose, 36 mg/L pyruvate, either warmed to 37 °C if the sample is immediately processed in the laboratory (within 30 min) or kept at 4 °C during longer transports (up to 24 h).
NOTE: Transportation solutions include an array of commercially available organ transportation/perfusion solutions as well as simple buffers such as PBS and MEM34. - Cut a small piece of the ovarian tissue (2 mm x 2 mm x2 mm) from the surface of the ovary. This piece contains the cortex, which is the outer portion of the ovary where the ovarian follicles are found35.
CAUTION: Handle human tissues such as the ovary using Biosafety Level 2 practices and procedures.
NOTE: A specialized reproductive medicine unit clinically cryopreserves the rest of the ovarian tissue for potential future use in fertility restoration of the patients. - Place the tissue in 350 µL Bouin's solution at room temperature for 2 h or in 350 µL of 4% formaldehyde at 4 °C overnight, ensuring that the tissue is fully submerged. After incubation, wash the tissue with 70% ethanol three times.
- For Bouin's solution, wash until the yellow color disappears. Embed the tissue in paraffin wax36. Cut a minimum of 30 serial tissue sections with a thickness of 4 µm and place them on glass slides.
CAUTION: Handle Bouin's solution and formaldehyde inside a chemical hood and with proper protective equipment.
NOTE: Formaldehyde might lead to shrinkage-related artifacts in the tissue. Bouin's solution is therefore the preferred choice for preserving histology. However, Bouin's solution carries additional risks, leading several institutions to prefer the use of formaldehyde37.
- For Bouin's solution, wash until the yellow color disappears. Embed the tissue in paraffin wax36. Cut a minimum of 30 serial tissue sections with a thickness of 4 µm and place them on glass slides.
2. Hematoxylin and Eosin (H&E) staining
- Weigh 5 g of eosin Y powder into a 100 mL autoclaved glass bottle. Add 800 mL of 95% ethanol and 200 mL of deionized water to make a 0.5% eosin stock solution. Prepare a 0.25% eosin working solution by diluting 200 mL of the 0.5% eosin stock solution with 160 mL of 100% ethanol, 1 mL of acetic acid, and 40 mL of deionized water.
NOTE: The powder may not dissolve completely. Filter the solution before use to reduce precipitate. - Place slides in racks. Immerse the rack with slides into the corresponding containers in the following order: 15 min in xylene, 5 min in 100% ethanol, dip 10 times in 70% ethanol, dip 10 times in 50% ethanol, and then dip 10 times in deionized water.
- Immerse the rack with slides in hematoxylin solution for 3 min, then transfer the rack with slides to a container with distilled tap water for 30 s to wash away excess hematoxylin stain.
- Place the rack with slides into a container with differentiation solution (1 mL of 37% HCl added to 100 mL of 70% ethanol) for 1 min, then transfer the rack with slides to a container with distilled tap water for 30 s.
NOTE: Ensure that the tissue sections are submerged in the liquid. Add more reagent to the container if needed. Change xylene after every 5 uses when staining large amounts. Prepare 50% ethanol by diluting 70% ethanol (e.g., add 35 mL of 70% ethanol to 15 mL of DPBS to make 50 mL of 50% ethanol).
CAUTION: Handle xylene inside a hood and with proper protective equipment.
- Place the rack with slides in Scott's tap water (also known as bluing solution) for 30-60 s, then transfer the rack with slides to a container with distilled tap water for 30 s. Immerse the rack with slides in the eosin working solution for 1 min.
- Dehydrate the slides by placing them in the following reagents: 100% ethanol for 30 s, a second container with 100% ethanol for 30 s, and then dip twice in xylene. After removing the rack from the xylene solution, let it dry in a glass container to prevent xylene from dripping inside the hood.
- Mount the slide with 2 drops of mounting solution. Carefully place the cover slides to avoid bubbles and let the mounting solution dry.
CAUTION: Handle xylene inside a hood and with proper protective equipment.
- Digitize slides by scanning them using slide scanners if possible. If this option is not available, use a regular inverted microscope at 20-40x magnification to directly count follicles. Ensure to take an image of the entire biopsy to facilitate area measurement in image analysis software.
3. Follicle counting
- If slides were digitized, open the files using appropriate software (e.g., open-source software QuPath38). If not digitized, perform the analysis directly using an inverted microscope and its respective software.
- Drag the file onto QuPath's main window. Set the image type to Brightfield H&E (see Figure 1A). Define 1 mm from the surface epithelium (a single layer of cuboidal cells lining the surface) using the scale bar. Click on the ellipse tool and annotate the follicles (see Figure 1B).
- Count the number of follicles within this 1 mm area, from the primordial to primary stage. Identify the stage of each follicle (primordial - a layer of flattened granulosa cells surrounding the oocyte; intermediary stage - a mixture of flattened and cuboidal granulosa cells; primary - one full layer of cuboidal granulosa cells surrounding the oocyte).
- Input the sample ID, age, number of follicles for each slide, section thickness, and nth section counted into the sheet named Follicle in mm3 on the Follicle Z-score calculator Excel file (see Supplementary File 1 and Figure 2A).
NOTE: Due to the diameter of follicles (smallest follicles >35 µm), the same follicle might appear in consecutive sections. Exercise extra caution when counting sections where the distance between sections is less than the follicle diameter. Preferably, count follicles from every 10th section (e.g., section #10, #20, #30). The surface epithelium may detach during tissue processing and might only be partially present in the slides. Count only follicles with visible cytoplasm. For quality control, have two people independently count the follicles and determine how well their counts align. Calculate inter-observer concordance using a simple correlation function between the follicle counts of each observer for the same sample.
- Identify the largest 10% of the follicles in each counted section by eyeballing. Measure the mean diameter of these oocytes by clicking on the line tool, drawing two perpendicular lines through the oocyte membrane, and selecting Measure, then Show annotation measurements (see Figure 1C).
- Record the measurements under the column Length µm (see Figure 1D). Input the mean diameter of all oocytes into the Mean oocyte diameter (µm) field for each slide in the Follicle Z-score calculator Excel file (Follicle in mm3, see Supplementary File 1 and Figure 2A).
- Place the image in the center of the screen in QuPath, then click on the ImageJ icon. Select Send snapshot to ImageJ (see Figure 3A). In ImageJ, demarcate 1 mm from the border using the brush tool. Click on the Image, then Type, and select 8-bit (see Figure 3B).
- Click on the line tool, draw a line on the scale bar at the lower left of the screen (see Figure 3C). Click on Analyze, then Set Scale, input the length indicated in the scale bar in the Known Distance field, and click on OK (see Figure 3C). Click on Image, then Adjust, Brightness/Contrast, and select Auto (see Figure 3D).
- Click on Image, then Adjust, Threshold, and select Auto (see Figure 3E). Click on the wand tool, select the area, then press Ctrl + M (see Figure 3F). Input the area value into the Follicle Z-score calculator Excel file (Follicle in mm3, see Supplementary File 1 and Figure 2A).
4. Follicle density, cortical volume, and Z-score calculations
- Use the Follicle Z-score calculator Excel file provided to automatically perform the calculations outlined below. The results will appear in the sheet "Z-score (follicle per mm³)" in the Excel calculator (see Supplementary File 1), provided that the details indicated in section 3 are entered into the sheet "follicles in mm³."
- To calculate follicle densities in these sections, use the modified formula presented by Schmidt et al.32. First, apply the correction factor α to account for double counting of the same follicle. The modified formula is as follows:
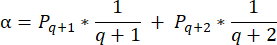
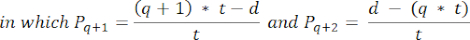
NOTE: In this formula, t is the section thickness, d is the diameter of the oocyte, and q is the quotient of the follicle diameter divided by section thickness. - Extrapolate the total number of follicles (n follicles) in the cortex from the follicle count in the section using the following formula33:
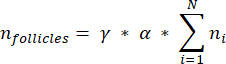
NOTE: This formula also accounts for all of the non-calculated sections. Here γ represents the number of sections that are not calculated (e.g., γ = 10 if every 10th section is counted), α is the correction factor calculated in 4.1, N is the number of sections counted, and n i is the total number of follicles in the counted sections.- Extrapolate the whole cortical volume (V) from the counted areas using the following formula33:
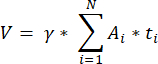
NOTE: Here, Ai is the area of the section, and ti is the tissue thickness. Please check step 4.2 for γ and N. - Use the following formula33 to calculate the follicle density:
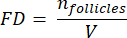
- Extrapolate the whole cortical volume (V) from the counted areas using the following formula33:
- Determine if there is reduced (<-1.7 Z-score) ovarian reserve by calculating the Z-score using the formula33:
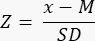
NOTE: The Z-score is automatically calculated in the sheet "Z-score (follicle per mm³)" in the Excel calculator (see Supplementary File 1) based on information provided in Section 3 (Follicle Counting) (Figure 2B). In this formula, Z represents the Z-score, x is the calculated follicle density (follicles/cm3) of the study sample (step 4.4), M is the mean follicle density of the respective age group, and SD is the standard deviation of the respective age group. Reference values for women aged 0-25 years have been established in Hassan et al.33. Means and standard deviations of follicle densities of the respective age groups can be found in the Follicle Z-score calculator Excel file (Supplementary File 1) and used for the Z-score calculations.
Results
Ovarian follicles are quantified after successful sectioning, staining, and scanning of slides. The different stages and health of the follicles are distinguished and further analyzed. In Figure 4A, an HE-stained cross-section of a Bouin-fixed ovary from an adult is displayed, with examples of cortical and medullary tissue. Figure 4B shows a representative picture of a child's ovary with an example of surface epithelium. The ovarian reserve (i.e., the non-gr...
Discussion
Women are born with a finite pool of oocytes, which cannot be replenished. Ovarian reserve is a key determinant of a woman's reproductive potential. This article outlines a method for the histological quantification of follicle density and its comparison to reference standards using Z-scores of ovarian cortical follicle densities in young patients and women up to 25 years of age.
In this protocol, ovarian tissue is obtained from girls and women at high or very high risk of infertility unde...
Disclosures
The authors have no conflicts of interest.
Acknowledgements
We would like to thank the healthcare professionals who helped us with participant recruitment and tissue collection and all the patients who donated their ovarian tissue. We also acknowledge the Childhood Cancer Fund (PR2020-0096) and Karolinska Institutet PhD funding KID, whose funding made this work possible. We would also like to thank the Morphological Phenotype Analysis facility for the preparation of the histological slides. We acknowledge Nicola Byers for language editing this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 1x Dulbecco's Phosphate Buffered Saline | Thermo Fisher | 14190144 | |
| 1x Phosphate Buffered Saline w/ CaCl2, MgCl2, 1 g/L D-glucose, 36 mg/L pyruvate | Thermo Fisher | 14287-080 | |
| 37% Hydrochloric acid | Sigma-Aldrich | 1.09057.1000 | |
| 70% ethanol | Histolabs | 01370.01L | |
| 95% ethanol | Fisher scientific | 10572143 | |
| Absolute ethanol | Histolabs | 01399.01L | |
| Bouin's solution | Sigma-Aldrich | HT10132-1L | |
| Eosin Y Free acid | Sigma-Aldrich | E4009-5G | |
| Formaldehyde | Thermo Fisher | 28908 | |
| Haematoxylin solution according to Delafield | Sigma-Aldrich | 03971-250ML | |
| ImageJ | |||
| Microsoft Excel | |||
| Microtome | |||
| Pertex mounting solution | Histolabs | 00840-05 | |
| Propofol | Baxter Holding B.V. | N01AX10 | ATC-code provided instead of catalog number |
| QuPath | https://qupath.github.io/ | ||
| Rocuronium bromide | B. Braun Melsungen AG | M03AC09 | ATC-code provided instead of catalog number |
| Scott's tap water substitute concentrate | Sigma-Aldrich | S5134-6x100ml | |
| Sevoflurane | Baxter Medical AB | N01AB08 | ATC-code provided instead of catalog number |
| Slide scanner | |||
| Sufentanil | Hameln Pharma gmbh | N01AH03 | ATC-code provided instead of catalog number |
| Sugammadex | Baxter Holding B.V. | V03AB35 | ATC-code provided instead of catalog number |
| SuperFrost plus white | Histolabs | 06400 | |
| SuperFrost Plus White microscope slides | Histolabs | 06400 | |
| Xylene | Saveen Werner | 131769.1611 |
References
- Richards, J. A. S. The ovarian cycle. Vitam Horm. 107, 1-25 (2018).
- Wallace, W. H. B., Kelsey, T. W. Human ovarian reserve from conception to the menopause. PLoS One. 5 (1), 1-9 (2010).
- Monniaux, D., et al. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link. Biol Reprod. 90 (4), 1-11 (2014).
- Gougeon, A., Trounson, A., Gosden, R., Eichenlaub-Ritter, U. . The early stages of follicular growth in biology and pathology of the oocyte. , 50-61 (2013).
- Gougeon, A. Human ovarian follicular development: From activation of resting follicles to preovulatory maturation. Ann Endocrinol. 71 (3), 132-143 (2010).
- Hansen, K. R., Craig, L. B., Zavy, M. T., Klein, N. A., Soules, M. R. Ovarian primordial and non-growing follicle counts according to the stages of reproductive aging workshop (STRAW) staging system. Menopause. 19 (2), 164-171 (2012).
- Gold, E. B. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 38 (3), 425-440 (2011).
- Alberico, H., et al. Workflow optimization for identification of female germline or oogonial stem cells in human ovarian cortex using single-cell RNA sequence analysis. Stem Cells. 40, 523-536 (2022).
- White, Y. A. R., et al. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 18 (3), 413-422 (2012).
- Wagner, M., et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun. 11, 1147 (2020).
- Yoshihara, M., et al. In reply: Revisiting claims of the continued absence of functional germline stem cells in adult ovaries. Stem Cells. 41 (2), 205-206 (2023).
- Yoshihara, M., et al. The continued absence of functional germline stem cells in adult ovaries. Stem Cells. 41, 105-110 (2023).
- Van Kasteren, Y. M., Schoemaker, J. Premature ovarian failure: A systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 5 (5), 483-492 (1999).
- McGlacken-Byrne, S. M., Conway, G. S. Premature ovarian insufficiency. Best Pract Res Clin Obstet Gynaecol. 81, 98-110 (2022).
- Viuff, M., Gravholt, C. H. Turner syndrome and fertility. Ann Endocrinol. 83 (4), 244-249 (2022).
- Poirot, C., et al. Ovarian tissue cryopreservation for fertility preservation in 418 girls and adolescents up to 15 years of age facing highly gonadotoxic treatment: Twenty years of experience at a single center. Acta Obstet Gynecol Scand. 98 (5), 630-637 (2019).
- Jensen, A. K., et al. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: Focus on pubertal development. Hum Reprod. 32 (1), 154-164 (2017).
- The Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril. 112 (6), 1022-1033 (2019).
- . ART Fact Sheet Available from: https://www.eshre.eu/Press-Room/Resources (2020)
- Antonouli, S., et al. A comprehensive review and update on human fertility cryopreservation methods and tools. Front Vet Sci. 10, 1151254 (2023).
- Bahroudi, Z., et al. Review of ovarian tissue cryopreservation techniques for fertility preservation. J Gynecol Obstet Hum Reprod. 51 (2), 102290 (2022).
- Jensen, A. K., et al. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: Focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 34 (3), 325-336 (2017).
- Donnez, J., Dolmans, M. -. M. Fertility preservation in women. N Engl J Med. 377 (17), 1657-1665 (2017).
- Dolmans, M. M., Falcone, T., Patrizio, P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil Steril. 114 (2), 279-280 (2020).
- Demeestere, I., et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 30 (9), 2107-2109 (2015).
- Ernst, E., Kjærsgaard, M., Birkebæk, N. H., Clausen, N., Andersen, C. Y. Case report: Stimulation of puberty in a girl with chemo- and radiation therapy-induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 49 (4), 911-914 (2013).
- Rodriguez-Wallberg, K. A., et al. Successful pregnancies after transplantation of ovarian tissue retrieved and cryopreserved at time of childhood acute lymphoblastic leukemia - a case report. Haematologica. 106 (10), 2783-2787 (2021).
- Matthews, S. J., Picton, H., Ernst, E., Andersen, C. Y. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 70 (4), 432-435 (2018).
- Kristensen, S. G., et al. Use of cryopreserved ovarian tissue in the Danish fertility preservation cohort. Fertil Steril. 116 (4), 1098-1106 (2021).
- Donfack, N. J., et al. Expectations and limitations of ovarian tissue transplantation. Zygote. 25 (4), 391-403 (2017).
- Schmidt, K. L. T., Byskov, A. G., Andersen, A. N., Müller, J., Andersen, C. Y. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 18 (6), 1158-1164 (2003).
- Hassan, J., et al. Reference standards for follicular density in ovarian cortex from birth to sexual maturity. Reprod Biomed Online. 47, 103287 (2023).
- Vilela, J. d. e. M. V., Dolmans, M. M., Amorim, C. A. Ovarian tissue transportation: A systematic review. Reprod Biomed Online. 42 (2), 351-365 (2021).
- Gibson, E., Mahdy, H. . Anatomy, abdomen and pelvis, ovary. , (2023).
- Nagaraj, A. S., et al. Establishment and analysis of tumor slice explants as a prerequisite for diagnostic testing. J Vis Exp. (141), e58569 (2018).
- Adeniran, B. V., Bjarkadottir, B. D., Appeltant, R., Lane, S., Williams, S. A. Improved preservation of ovarian tissue morphology that is compatible with antigen detection using a fixative mixture of formalin and acetic acid. Hum Reprod. 36 (7), 1871-1890 (2021).
- Bankhead, P., et al. QuPath: Open-source software for digital pathology image analysis. Sci Rep. 7 (1), 1-7 (2017).
- Björvang, R. D., et al. Persistent organic pollutants and the size of ovarian reserve in reproductive-aged women. Environ Int. 155, 106589 (2021).
- Kwok, R., Johnson, N. P. Ovarian biopsy has no role as a routine diagnostic test of ovarian reserve: A systematic review. Reprod Biomed Online. 24, 492-495 (2012).
- Ahmad, A., et al. High-throughput spatial sensitive, quantitative phase microscopy using low spatial and high temporal coherent illumination. Sci Rep. 11 (1), 1-13 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved