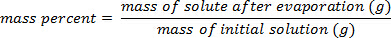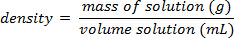Determining the Mass Percent Composition in an Aqueous Solution
Source: Laboratory of Dr. Neal Abrams — SUNY College of Environmental Science and Forestry
Determining the composition of a solution is an important analytical and forensic technique. When solutions are made with water, they are referred to as being aqueous, or containing water. The primary component of a solution is referred to as the solvent, and the dissolved minor component is called the solute. The solute is dissolved in the solvent to make a solution. Water is the most common solvent in everyday life, as well as nearly all biological systems. In chemistry labs, the solvent may be another liquid, like acetone, ether, or an alcohol. The solute can be a liquid or a solid, but this experiment only addressesthe determination of solids.
1. Percent by Mass - Direct
- Place a small volume of a solution into a clean and oven-dried beaker or crystallization dish.
- After accurately determining the precise total mass of the solution, heat the beaker or dish on a hotplate or in an oven to drive off the water. Slow evaporation is the best method, as boiling can result in splattering of the solution.
- Once the solvent has evaporated, cool the remaining solid (solute) and determine the mass.
- Calculate the mass percent as:
Using the example shown in Figure 1, a set of sodium chloride standards was prepared with mass percent compositions of 5.000%, 10.00%, 15.00%, 20.00%, and 25.00% of solute in solution. The measured densities were 1.025, 1.042, 1.060, 1.070, and 1.090 g/mL, respectively. After plotting these data, a linear trendline is applied, fitting the equation y = 3.446 x 10-3x + 1.0048, where y is the density and x is the mass percent composition.
Next, the volume o
The percentage of sugar in soda, could easily be determinedusing the principle of mass percent composition. The procedure for doing this experiment would be to measure the mass and volume of degassed soda (no bubbles) and to calculate the solution's density. A calibration curve of density vs. percent by mass for several standard sucrose (sugar) solutionswould need to be created, and then that calibration could be used to solve for the percent of sucrose in the soda. One assumption is that sucrose is the major contributor to a change in d
Vai a...
Video da questa raccolta:

Now Playing
Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.0K Visualizzazioni

Common Lab Glassware and Uses
General Chemistry
653.0K Visualizzazioni

Solutions and Concentrations
General Chemistry
272.8K Visualizzazioni

Determining the Density of a Solid and Liquid
General Chemistry
554.5K Visualizzazioni

Determining the Empirical Formula
General Chemistry
179.8K Visualizzazioni

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.0K Visualizzazioni

Using a pH Meter
General Chemistry
344.2K Visualizzazioni

Introduction to Titration
General Chemistry
423.2K Visualizzazioni

Ideal Gas Law
General Chemistry
78.0K Visualizzazioni

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.2K Visualizzazioni

Le Châtelier's Principle
General Chemistry
263.8K Visualizzazioni

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.4K Visualizzazioni

Determining Rate Laws and the Order of Reaction
General Chemistry
195.6K Visualizzazioni

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Visualizzazioni

Coordination Chemistry Complexes
General Chemistry
91.1K Visualizzazioni