Coordination Chemistry Complexes
Source: Laboratory of Dr. Neal Abrams — SUNY College of Environmental Science and Forestry
Transition metals are found everywhere from vitamin supplements to electroplating baths. Transition metals also make up the pigments in many paints and compose all minerals. Typically, transition metals are found in the cationic form since they readily oxidize, or lose electrons, and are surrounded by electron donors called ligands. These ligands do not form ionic or covalent bonds with the metal center, rather they take on a third type of bond known as coordinate-covalent. The coordinate-covalent bond between a ligand and a metal is dynamic, meaning that ligands are continuously exchanging and re-coordinating around the metal center. The identities of both the metal and the ligand dictates which ligands will bond preferentially over another. In addition, color and magnetic properties are also due to the types of complexes that are formed. The coordination compounds that form are analyzed using a variety of instruments and tools. This experiment explores why so many complexes are possible and uses a spectrochemical (color and chemical) method to help identify the type of coordination complex that forms.
1. Nickel Complexes and Colors
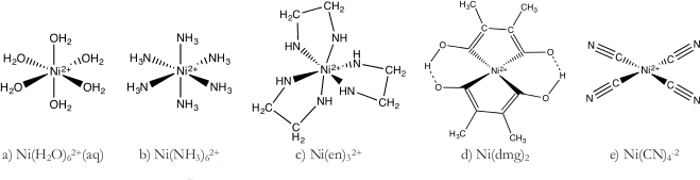
- Ni(H2O)62+ complex (Figure 1a)
- Prepare a 1 M solution of Ni(H2O)62+ by dissolving NiSO4 in the appropriate volume of water.
- Further dilute the Ni(H2O)62+solution by adding 70 mL of the 1 M solution to 1,000 mL of deionized water.
- Divide the Ni(H2O)62+ among seven 400-mL beakers.
From pigments to people, transitional metals are found throughout fields of chemistry, biology, geology, and engineering. Understanding the behavior of transition metals under different chemical states can be as simple as monitoring color or magnetic behavior. Nearly every 3d (4th row) transition metal is vital to physiological function and, in all cases, these metals are bound by ligands to form coordination complexes. For example, iron is vital to oxygen transport in all vertebrates. Hemoglobin, a complex pr
- Shakhashiri, B. Z.; G. E. Dirreen, G. E; Juergens, F. Color, Solubility, and Complex Ion Equilibria of Nickel (II) Species in Aqueous Solution. J. Chem. Ed. 52 (12), 900-901 (1980).
Vai a...
Video da questa raccolta:

Now Playing
Coordination Chemistry Complexes
General Chemistry
91.1K Visualizzazioni

Common Lab Glassware and Uses
General Chemistry
652.9K Visualizzazioni

Solutions and Concentrations
General Chemistry
272.7K Visualizzazioni

Determining the Density of a Solid and Liquid
General Chemistry
554.4K Visualizzazioni

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
382.9K Visualizzazioni

Determining the Empirical Formula
General Chemistry
179.6K Visualizzazioni

Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.0K Visualizzazioni

Using a pH Meter
General Chemistry
344.2K Visualizzazioni

Introduction to Titration
General Chemistry
423.1K Visualizzazioni

Ideal Gas Law
General Chemistry
77.9K Visualizzazioni

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.2K Visualizzazioni

Le Châtelier's Principle
General Chemistry
263.8K Visualizzazioni

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.4K Visualizzazioni

Determining Rate Laws and the Order of Reaction
General Chemistry
195.6K Visualizzazioni

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Visualizzazioni