A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Calcium Imaging in Mouse Superior Colliculus
* These authors contributed equally
In This Article
Summary
This protocol details the procedure for imaging calcium responses in the superior colliculus (SC) of awake mice, including imaging single-neuron activity with two-photon microscopy while leaving the cortex intact in wild-type mice, and imaging the entire SC with wide-field microscopy in partial-cortex mutant mice.
Abstract
The superior colliculus (SC), an evolutionarily conserved midbrain structure in all vertebrates, is the most sophisticated visual center before the emergence of the cerebral cortex. It receives direct inputs from ~30 types of retinal ganglion cells (RGCs), with each encoding a specific visual feature. It remains elusive whether the SC simply inherits retinal features or if additional and potentially de novo processing occurs in the SC. To reveal the neural coding of visual information in the SC, we provide here a detailed protocol to optically record visual responses with two complementary methods in awake mice. One method uses two-photon microscopy to image calcium activity at single-cell resolution without ablating the overlaying cortex, while the other uses wide-field microscopy to image the whole SC of a mutant mouse whose cortex is largely undeveloped. This protocol details these two methods, including animal preparation, viral injection, headplate implantation, plug implantation, data acquisition, and data analysis. The representative results show that the two-photon calcium imaging reveals visually evoked neuronal responses at single-cell resolution, and the wide-field calcium imaging reveals neural activity across the entire SC. By combining these two methods, one can reveal the neural coding in the SC at different scales, and such combination can also be applied to other brain regions.
Introduction
The superior colliculus (SC) is an important visual center in all vertebrates. In mammals, it receives direct inputs from the retina and the visual cortex1. While optical recording has been widely applied to the cortex2,3,4,5, its application in the SC is hindered by poor optical accesss6,7,8,9,10,11,12,13,14,15,16,17,18,19. The goal of this protocol is to provide details about two complementary methods for optical recording of the neural activity in the SC.
The SC is located beneath the cortex and transverse sinus, which limits optical access to the collicular neurons. One approach to overcome this limitation is to aspirate the overlaying cortex and expose the anterior-lateral SC7,9,10,13,14,19. However, because the SC receives cortical inputs, such an operation might affect how the SC neurons respond to visual stimuli. To overcome this limitation, we detail here an alternative protocol to image the superficial layer of the posterior-medial SC with a silicon plug, while leaving the cortex intact8,11. Specifically, to achieve single-cell resolution, we applied two-photon microscopy to image calcium responses in the posterior-medial SC of wild-type mice. In addition, to achieve broad coverage, we applied wide-field microscopy to image the entire SC of a mutant mouse whose posterior cortex has not developed20.
The two methods described in this protocol are complementary to each other. The two-photon calcium imaging without ablating the cortex is appropriate for recording neural activity at single-cell resolution with intact cortical inputs. The wide-field calcium imaging is appropriate for recording neural activity in the entire SC while sacrificing spatial resolution.
Protocol
All experimental procedures were performed in accordance with the animal welfare guidelines and approved by the IACUC at the Chinese Institute for Brain Research, Beijing.
NOTE: The timeline for this protocol is as follows: 1) make the suction cup; 2) inject the virus; 3) implant the headplate; 4) after 3 weeks, implant the plug; 5) after a ~3 day recovery and habituation on the treadmill, perform two-photon/wide-field imaging.
1. Preparation of a suction cup (Figure 1A)
- Deposit a drop of phosphate-buffered saline (PBS, 1x) in an acrylic dish and touch it with a 21 G flat needle to fill the tip by capillarity.
- Cover the tip of the needle with a translucent silicone adhesive and set for about ~10 min.
- Cut the silicone adhesive with scissors to a disk with a ~2 mm diameter.
2. Preparation of plugs (Figure 1B)
- Prepare a 0.75 mm thick plastic shim stock and cut out a 1 cm x 1 cm square in the center. Prepare two acrylic blocks. Clean the shim stock and acrylic blocks with alcohol and wipe them with lens paper.
- Put the shim stock on one acrylic block, deposit the silicone adhesive into the center to fill ~90% of the opening (avoid bubbles), add another acrylic block and ~1 kg force, and wait 20 min.
- Under a surgical microscope, cut the silicone adhesive with a scalpel to 1 mm high by 1.5 mm wide triangular prisms above a piece of paper with triangles printed on it.
- Remove any dust from the triangular prisms with plastic sticky tape and put it on a glass coverslip of 5 mm diameter and 0.15 mm thickness.
- Put the coverslip with the triangular prisms under a corona treater, turn on the corona treater, and bring it closer to the coverslip until lightening appears. Hold it at that position for a few minutes until they are attached to each other.
NOTE: This step might be different for other corona treaters. - Put the plugs in a Petri dish and place it in an incubator at 70 °C overnight.
3. Preparation of animals and injection of viral construct
- Use C57BL/6J (wild-type) and Emx1-Cre:Pals1flox/wt (partial-cortex)20 mice of both sexes at 6 weeks of age (9 weeks at the time of imaging).
- Sterilize all materials and instruments used for the surgical procedure.
- Anesthetize the mouse with 5% isoflurane and then maintain the isoflurane at 1%-2% with a flow rate of 1 L/min during the surgery. Confirm the level of anesthesia by the lack of toe pinch reflex.
- Head-fix the mouse on a stereotaxic frame with ear bars. Throughout the surgery, put ophthalmic ointment on the mouse eyes to prevent drying, and maintain its body temperature at 37 °C with a thermostatic heating pad.
- Shave the fur and sterilize the skin surface with iodophor and 75% ethanol. Inject lidocaine (5 mg/kg, subcutaneous injection [SQ]), tilidine (2 mg/kg, SQ), and meloxicam (2 mg/kg, SQ).
NOTE: The surgical procedure for sterilization, analgesia, and local anesthesia may differ across institutions. - Remove the skin over the SC with surgical scissors to expose the skull. Clean the skull with a cotton swab.
- Adjust the stereotaxic frame until the skull surface is flat. Drill a hole 0.5 mm lateral and 0.42 mm anterior to the lambda until the dura is exposed.
- Inject adeno-associated virus (AAV) expressing GCaMP6m (rAAV2/9-hsyn-GCaMP6m; 1 x 1013 GC/mL dissolved in 1x PBS) at depths of 1 mm and 1.6 mm from the lambda with a beveled glass micropipette (tip is beveled to 25° with a diameter of 40-50 µm).
- At each depth, inject 100 nL at a speed of 50 nL/min and wait 5 min after each injection. After the injection, slowly withdraw the injector.
4. Implantation of the headplate
- Clean the muscles and tissues over the skull and scratch the skull with a blade. If bleeding occurs, stop it with gel foam and clean the blood. Attach the headplate (Figure 1C) to the skull with a self-curing dental adhesive resin cement. Specifically, mix a 3/4 spoon of polymer, three drops of monomer, and one drop of catalyst in a ceramic bowl on ice. Apply the mixture on the headplate and the skull. Attach them together and wait 5 min for the adhesive to set.
- Inject antibiotics (ceftiofur sodium, 5 mg/kg, SQ) to prevent infections. Remove the mouse from the stereotaxic frame and place it on a heating pad for recovery. Return it to the home cage after recovering from anesthesia. For pain management, provide ibuprofen-containing drinking water (0.5 mL/50 mL) to the mouse for 3 days after the surgery.
5. Implantation of the plug (Figure 2)
- Implant the plug 3 weeks after the viral injection. Anesthetize and fix the mouse on a stereotaxic frame, as described in steps 3.2-3.5. Prepare gel foam soaked in saline to stop potential bleeding.
- Drill a 3 mm x 2 mm oval with a microdrill centered at 0.5 mm posterior to the lambda. Thin the boundary of the oval until it cracks. Thin the skull in front of the oval recording window to make the coverslip close to the SC.
- Take off bone flaps using fine forceps. Make a cut on the dura posterior to the transverse sinus and remove the piece of dura. If necessary, add artificial cerebrospinal fluid (ACSF) to the brain with a 3 mL syringe to prevent drying. Dry the skull and recording window with gel foam and cotton swabs.
- Deposit a drop of silicone adhesive into the recording window. Use the suction cup to hold the plug with negative pressure. Use a motorized micromanipulator to move the suction cup and lower the plug into the silicone adhesive until the coverslip touches the skull. Then, move the plug forward ~1 mm to push away the transverse sinus and expose the SC. This step should be done in 1-2 min before the silicone adhesive becomes sticky.
- Clean the headplate and apply butyl cyanoacrylate and resin cement around the boundary of the plug to fix it to the headplate.
NOTE: Provide postoperative care, as described in step 4.2.
6. Two-photon imaging (Figure 3)
- Head-fix the animal on a rotating treadmill with its headplate. Place the treadmill under a two-photon microscope and adjust the height to an appropriate position. Put a black aluminum cone between the objective and the headplate to prevent light contamination from the monitor for visual stimulation. Habituate the mouse for ~15 min.
- Perform two-photon imaging on the microscope with a 16x magnification, 0.8 numerical aperture (NA), and 3 mm working distance (WD) objective. Use a Ti:sapphire laser with mode-locking technique controlled by two galvo scanners to excite GCaMP6m at 920 nm. Adjust the laser power at the sample plane between 20-80 mW.
- Scan a 600 µm x 600 µm field of view at 4.8 Hz with a spatial resolution of 2.4 µ m/pixel, and image neural activity across the depth up to 350 µ m.
NOTE: The sampling rate and spatial resolution are for galvo scanners, and should be adjusted for resonant scanners. - Collect emitted fluorescence with a dichroic mirror and detect it using a photomultiplier tube (PMT) after passing it through a bandpass filter.
- Record the mouse's locomotion on the treadmill with a rotary encoder. Use a camera with a 50 mm lens to record its pupil size and position. Synchronize these recordings and image acquisitions to visual stimulation by recording triggers sent by the stimulation computer.
7. Analysis of calcium responses measured by two-photon imaging
- Correct the brain motion during imaging using SIMA21 or NoRMCorre22.
- Manually draw a region of interest (ROI) using the Cell Magic Wand tool (ImageJ) and fit it with an ellipse in MATLAB. Extract fluorescence traces of each ROI after removing neuropil contamination:
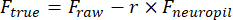
Ftrue and Fraw are the corrected and raw fluorescence amplitudes of the ROI, respectively, Fneuropil is the fluorescence amplitude of the surrounding neuropil, and r is the out-of-focus neuropil contamination factor (0.7 for our setup). The r is estimated by measuring the signals on a blood vessel for which Ftrue = 0, as described previously23,24. - Remove slow baseline fluctuations by subtracting the eighth percentile value from a 15 s window centered on each frame25.
- Define visual responses of a neuron as the relative change of fluorescence amplitude in its ROI during the stimulus period:
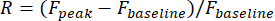
Fpeak is the peak amplitude of the fluorescence trace during the visual stimulation, and Fbaseline is the mean amplitude during a 0.5 s period prior to stimulation.
8. Wide-field imaging and data analysis (Figure 4)
- Build a wide-field macroscope with a tandem lens4 (Figure 4A). The tandem-lens macroscope is two camera lenses (50 mm and 105 mm) connected via an adapter, and the magnification is ~2x.
- Prepare partial-cortex mutant mice as described in steps 3.2-3.5. Inject 100 nL of AAV expressing GCaMP6m at a depth of 200 µm at the center of each side of the SC.
- After ~3 weeks, implant a 5 mm diameter coverslip over the SC. Perform an oval craniotomy of 4 mm x 3 mm centered at the SC and thin the bone around it. Then, press the coverslip directly onto the SC and fix it with resin cement.
- Excite GCaMP6m with a blue light emitting diode (LED) with an excitation filter (469 nm ± 35 nm). Acquire images using a complementary metal oxide semiconductor (CMOS) camera after passing an emission filter (525 nm ± 39 nm) at 10 Hz. The camera covers an area of 5.36 mm x 2.85 mm at a spatial resolution of 2.63 µm/pixel.
- Convolve each acquired image with a normalized box filter and downsample it to 1/4 of the original size. For each pixel in the image, define the response, as described in step 7.4.
NOTE: Provide postoperative care, as described in step 4.2.
Results
Figures 1A,B show how to make the suction cup and the plugs, respectively. Figure 2 shows how to implant the plug successfully. After implanting the plug, the posterior-medial SC is exposed, as shown in Figure 2D. Figure 3 shows calcium responses of SC neurons from an example wild-type mouse imaged using two-photon microscopy. The triangular prism, which is easily captured under th...
Discussion
Critical steps in the protocol
The most critical step is the craniotomy in steps 5.2 and 5.3. First, the bone at 0.5 mm posterior to the lambda is thick and has blood vessels inside, which can cause bleeding during the drilling process. Adequate gel foam should be prepared to stop the bleeding. Second, there is a good chance of angiorrhexis when removing the bone just above the transverse sinus. For troubleshooting, one alternative approach is to thin the bone inside the oval and remove it piece by...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (32271060). Y.-t.L. designed the research, performed the experiment, analyzed the data, and wrote the manuscript. Z.L. and R.W. performed the experiment.
Materials
| Name | Company | Catalog Number | Comments |
| 16x objective | Nikon | ||
| 50-mm lens | Computar | M5018-MP2 | |
| 5-mm coverslip | Warner instruments | CS-5R | |
| bandpass filter | Chroma Technology | HQ575/250 m-2p | |
| butyl cyanoacrylate | Vetbond, World Precision Instruments | ||
| camera for monitoring pupil | FLIR | BFS-U3-04S2M-CS | |
| camera for widefield imaging | Basler | acA2000-165µm | |
| corona treater | Electro-Technic Products | BD-20AC | |
| dichroic | Chroma Technology | T600/200dcrb | |
| galvanometers | Cambridge Technology | ||
| glass bead sterilizer | RWD | RS1502 | |
| microdrill | RWD | 78001 | |
| micromanipulator | Sutter Instruments | QUAD | |
| photomultiplier tube | Hamamatsu | R3896 | |
| rotory encoder | USdigital | MA3-A10-125-N | |
| self-curing dental adhesive resin cement | SuperBond C&B, Sun Medical Co, Ltd. Moriyama, Japan | ||
| thermostatic heating pad | RWD | 69020 | |
| Ti:Sapphire laser | Spectra-Physics | Mai Tai HP DeepSee | |
| translucent silicone adhesive | Kwik-Sil, World Precision Instruments | ||
| treadmill | Xinglin Biology | ||
| Virus Strains | |||
| rAAV2/9-hsyn-Gcamp6m | Vector Core at Chinese Institute for Brain Research, Beijing | ||
| Animals | |||
| C57BL/6J wild type | Laboratory Animal Resource Center at Chinese Institute for Brain Research, Beijing | ||
| Emx1-Cre | The Jackson Laboratory | 5628 | |
| Pals1flox/wt | Christopher A. Walsh Lab | ||
| Software | |||
| ImageJ | NIH Image | ||
| Labview | National Instruments | ||
| MATLAB | Mathworks |
References
- May, P. J. The mammalian superior colliculus: laminar structure and connections. Progress in Brain Research. 151, 321-378 (2006).
- Denk, W., Strickler, J. H., Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science. 248 (4951), 73-76 (1990).
- Ohki, K., Chung, S., Ch'ng, Y. H., Kara, P., Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 433 (7026), 597-603 (2005).
- Ratzlaff, E. H., Grinvald, A. A tandem-lens epifluorescence macroscope: Hundred-fold brightness advantage for wide-field imaging. Journal of Neuroscience Methods. 36 (2-3), 127-137 (1991).
- de Vries, S. E. J., et al. A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nature Neuroscience. 23 (1), 138-151 (2020).
- Mrsic-Flogel, T. D., et al. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. The Journal of Neuroscience. 25 (29), 6921-6928 (2005).
- Cang, J., Wang, L., Stryker, M. P., Feldheim, D. A. Roles of ephrin-as and structured activity in the development of functional maps in the superior colliculus. The Journal of Neuroscience. 28 (43), 11015-11023 (2008).
- Feinberg, E. H., Meister, M. Orientation columns in the mouse superior colliculus. Nature. 519 (7542), 229-232 (2015).
- Ahmadlou, M., Heimel, J. A. Preference for concentric orientations in the mouse superior colliculus. Nature Communications. 6, 6773 (2015).
- de Malmazet, D., Kühn, N. K., Farrow, K. Retinotopic separation of nasal and temporal motion selectivity in the mouse superior colliculus. Current Biology. 28 (18), 2961-2969 (2018).
- Li, Y. T., Turan, Z., Meister, M. Functional architecture of motion direction in the mouse superior colliculus. Current Biology. 30 (17), 3304-3315 (2020).
- Gribizis, A., et al. Visual cortex gains independence from peripheral drive before eye opening. Neuron. 104 (4), 711-723 (2019).
- Inayat, S., et al. Neurons in the most superficial lamina of the mouse superior colliculus are highly selective for stimulus direction. The Journal of Neuroscience. 35 (20), 7992-8003 (2015).
- Barchini, J., Shi, X., Chen, H., Cang, J. Bidirectional encoding of motion contrast in the mouse superior colliculus. eLife. 7, 35261 (2018).
- Savier, E. L., Chen, H., Cang, J. Effects of locomotion on visual responses in the mouse superior colliculus. The Journal of Neuroscience. 39 (47), 9360-9368 (2019).
- Schröder, S., et al. Arousal modulates retinal output. Neuron. 107 (3), 487-495 (2020).
- Ge, X., et al. Retinal waves prime visual motion detection by simulating future optic flow. Science. 373 (6553), (2021).
- Chen, H., Savier, E. L., DePiero, V. J., Cang, J. Lack of evidence for stereotypical direction columns in the mouse superior colliculus. The Journal of Neuroscience. 41 (3), 461-473 (2021).
- Kasai, M., Isa, T. Effects of light isoflurane anesthesia on organization of direction and orientation selectivity in the superficial layer of the mouse superior colliculus. The Journal of Neuroscience. 42 (4), 619-630 (2022).
- Kim, S., et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron. 66 (1), 69-84 (2010).
- Kaifosh, P., Zaremba, J. D., Danielson, N. B., Losonczy, A. S. I. M. A. Python software for analysis of dynamic fluorescence imaging data. Frontiers in Neuroinformatics. 8, 80 (2014).
- Pnevmatikakis, E. A., Giovannucci, A. NoRMCorre: An online algorithm for piecewise rigid motion correction of calcium imaging data. Journal of Neuroscience Methods. 291, 83-94 (2017).
- Kerlin, A. M., Andermann, M. L., Berezovskii, V. K., Reid, R. C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 67 (5), 858-871 (2010).
- Göbel, W., Helmchen, F. In vivo calcium imaging of neural network function. Physiology. 22 (6), 358-365 (2007).
- Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L., Tank, D. W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 56 (1), 43-57 (2007).
- Evans, D. A., et al. A synaptic threshold mechanism for computing escape decisions. Nature. 558 (7711), 590-594 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved