A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Practical Considerations for the Design, Execution, and Interpretation of Studies Involving Whole-Bone Bending Tests of Rodent Bones
In This Article
Summary
Mechanical testing of rodent bones is a valuable method for extracting information regarding a bone's susceptibility to fracture. Lacking proper practical understanding, the results may be overinterpreted or lack validity. This protocol will serve as a guide to ensure mechanical tests are performed accurately to provide valid and functional data.
Abstract
Skeletal fragility leading to fracture is an American public health crisis resulting in 1.5 million fractures each year and $18 billion in direct care costs. The ability to understand the mechanisms underlying bone disease and the response to treatment is not only desired, but critical. Mechanical testing of bone serves as a valuable technique for understanding and quantifying a bone's susceptibility to fracture. While this method appears simple to perform, inappropriate and inaccurate conclusions may be reached if governing assumptions and key steps are disregarded by the user. This has been observed across disciplines as studies continue to be published with misuse of methods and incorrect interpretation of results. This protocol will serve as a primer for the principles associated with mechanical testing along with the application of these techniques-from considerations of sample size through tissue harvesting and storage, to data analysis and interpretation. With this in hand, valuable information regarding a bone's susceptibility to fracture may be obtained, furthering understanding for both academic research and clinical solutions.
Introduction
Mechanical testing of bone is the primary method to extract functional information related to a bone's susceptibility to fracture. In preclinical studies, several testing modalities can be used but by far the most common is the bending of long bones. These tests are easy to perform and can be used on bones ranging in size from human to mouse. As mice are one of the most commonly studied animals in preclinical research, this protocol will focus on bending tests performed on the femora and tibiae of mice.
Prior to performing bending tests, bones must be properly harvested and stored. The most common storage methods have traditionally been freezing bones in saline-soaked gauze, freezing in saline alone, or dehydrating bones in ethanol 1. Bones stored in ethanol have been shown to have increased stiffness and elastic modulus and decreased deformation parameters versus those stored frozen1. Even rehydrating the bones prior to testing does not recover these properties back to normal levels 1. Storing submerged in saline could cause damage to the bone since pressure is exerted as the saline expands. In addition, a complete thaw of the solution would be required to remove the bones for microcomputed tomography (µCT) scanning. Consequently, freezing freshly harvested bones in saline-soaked gauze has become the standard storage method and is recommended throughout this protocol.
Because the size and shape of a bone affect its bulk strength and many disease models significantly alter bone size and morphology, engineering principles are used to normalize away the effects of size to produce properties that estimate the behavior of the tissue2. This approach requires cross-sectional geometry of the failure location, which is most commonly acquired using µCT to create scans of the bones prior to testing. µCT is widely used due to its availability and high image resolution. Moreover, contributions of soft tissue are not included, and scanning does not require chemical fixation or other modifications to the bone3,4. In all forms of CT, an X-ray source is focused on an object while a detector on the other side of the object measures the resulting X-ray energy. This produces an X-ray shadow of the sample that can be converted into an image3,5. The object being scanned is rotated (or the X-ray source and detector are rotated around the sample), generating images that can be reconstructed into a three-dimensional data set representing the object5.
Scan resolution, or how close together two objects can be and still be resolved individually, is controlled by changing the nominal voxel size or the size of a pixel in the resulting image. It is generally accepted that objects must be at least two times the size of a single voxel to be identified3, but a higher ratio will allow for improved precision. Further, larger voxels are more prone to partial volume effects: when a single voxel contains tissues of varying densities, it is assigned the average of these densities, rather than the specific density of a single tissue, which may lead to an over- or under-estimation of tissue areas and mineral density3. While these issues can be mitigated by choosing smaller voxel sizes, using a higher resolution does not ensure the elimination of partial volume effects and may require longer scan times3. When scanning bones ex vivo, a voxel size of 6-10 µm is generally recommended to accurately assess the trabecular architecture of mouse bones. A larger voxel size of 10-17 µm can be used for cortical bone, although the smallest reasonable voxel size should be used. This protocol uses a 10 µm voxel size, which is small enough to differentiate key trabecular properties and minimize partial volume effects without extensive scan time.
X-ray energy and energy filter settings must also be selected carefully, as the high mineral density and thickness of bone tissue greatly attenuates and alters the transmitted X-ray energy spectrum. It is generally assumed that because the emitted X-ray spectrum is equivalent to the spectrum that exits the object6, using low-energy X-rays on dense objects such as bone can lead to an artifact known as beam hardening7. A higher voltage of 50-70 kVp is recommended when scanning bone samples to reduce the incidence of these artifacts5. In addition, inserting an aluminum or copper energy filter creates a more concentrated energy beam, further minimizing artifacts4,7. A 0.5 mm aluminum filter will be used throughout this protocol.
Finally, the scan rotation step and rotation length (e.g., 180°-360°), together control the number of images captured, which determines the amount of noise in the final scan4. Averaging multiple frames in each step can reduce noise but may increase scan time4. This protocol uses a rotation step of 0.7 degrees and a frame averaging of 2.
One final note about scanning: hydroxyapatite calibration phantoms should be scanned using the same scan settings as the experimental bones to enable the conversion of attenuation coefficients to mineral density in g/cm35. This protocol uses phantoms of 0.25 g/cm3 and 0.75 g/cm3 of hydroxyapatite, although different phantoms are available. Note that some scanning systems use internal phantoms as part of daily system calibration.
Once scanning is complete, the angular projections are reconstructed into cross-sectional images of the object, typically using the manufacturer's accompanying software. Whatever system is used, it is important to ensure that the whole bone is captured in the reconstruction and that thresholding is set appropriately to allow for the recognition of bone versus non-bone. After reconstruction, it is critical to rotate all scans in three dimensions so that bones are oriented consistently and properly aligned with the transverse axis, again using the manufacturer's software.
Following rotation, regions of interest (ROI) for analysis may be selected based on whether cortical properties, trabecular properties, or fracture geometry for mechanical normalization are desired. For the latter, ROIs should be selected after testing by measuring the distance from the fracture site to one end of the bone and using voxel size to determine the corresponding slice location in the scan file. The selected region should be at least 100 µm in length, with the fracture point at the approximate center of the ROI, to provide adequate estimation4.
With ROIs selected, two properties are needed for mechanical normalization (to calculate bending stress and strain): the maximum distance from the neutral bending axis to the surface where failure is initiated (assumed to be the surface loaded in tension, determined by the testing setup), and the area moment of inertia around the neutral axis, (also dependent on testing setup). This protocol recommends the use of a custom code to determine these values. For access to the code, contact the corresponding author directly or visit the lab website at https://bbml.et.iupui.edu/ for more information.
Once µCT scanning has been completed, mechanical testing can begin. Bending tests can be performed in either four-point or three-point configurations. Four-point bending tests are preferred as they eliminate shear stress in the bone between loading points, allowing for pure bending to occur in this region3. The bone will then fracture due to tension, creating a failure that is more representative of the true bending properties of the bone3. However, the bone must be loaded in such a way as to deliver the same load at both loading points (this can be facilitated with a pivoting loading head). In three-point bending tests, there is a large change in shear stress where the load point meets the bone, which causes the bone to break at this point due to shear, not tension3. ASTM standards recommend materials undergoing bending should have a length-to-width ratio of 16:1, meaning the length of the support span should be 16 times larger than the width of the bone to minimize impacts of shear8,9. This is often impossible to achieve when testing small rodent bones, so the loading span is simply made as large as possible but with as small of a change in cross-sectional shape as possible. Moreover, when performing four-point bending, the ratio between the lengths of the lower and upper span should be ~3:18, which can usually be achieved in the tibia, but it is difficult in the shorter femur. In addition, the thinner cortical walls of femurs make them susceptible to ring-type deformation which changes the shape of the bone cross-section during the test (this can be accentuated in four-point tests as a greater force is required to induce the same bending moment compared to three-point bending). Therefore, three-point bending will be utilized for mouse femora while four-point bending will be used for tibiae throughout this protocol.
Finally, it is important to properly power the study for statistical analysis. A general recommendation for mechanical testing is to have a sample size of 10-12 bones per experimental group to be able to detect differences, as some mechanical properties, especially postyield parameters, can be highly variable. In some cases, this may mean starting with a higher animal sample size given attrition that could occur during the study. Sample size analysis using existing data should be completed prior to attempting a study.
There are numerous limitations and assumptions, but bending tests can provide quite accurate results, especially when relative differences between groups are of interest. These properties, together with the analysis of trabecular architecture and cortical morphology, can provide better insight into disease states and treatment regimens. If care is taken with those aspects of the experiment that are in our control (e.g., harvesting, storing, scanning, and testing), we can feel confident that accurate results have been generated.
Protocol
All procedures described throughout this protocol that involved animals have been approved by the Indiana University School of Science Institutional Animal and Use Committee (IACUC) prior to the procedure. Animals described in the procedure were euthanized via CO2inhalation followed by cervical dislocation as a secondary means of euthanasia.
1. Harvest, storage, and thawing of bones
- Harvest and storage
- Place the mouse ventral side up. Use a scalpel (or a razor blade or scissors) to make an incision at the approximate junction of the femur and pelvis on one side.
- Continue the initial incision dorsally until the hip joint is located; look for the femoral head that appears as a small white sphere attached to the pelvis.
- Apply pressure with the edge of a scalpel to the proximal edge of the femoral head until the femoral head pops out of the socket. Excise additional tissue to free the hindlimb from the remainder of the carcass.
- With the hindlimb isolated, separate the tibia and femur by inducing flexion at the knee joint. Move the scalpel in the medial-lateral direction at the anterior surface of the knee to cut through any adjoining tissue, including the ligaments between the bones.
- If this does not separate the bones, extend the knee joint to allow access to the posterior surface. Be careful to avoid cutting the bone or scraping the articular cartilage.
- Once the femur and tibia are separated, remove the hindfoot from the tibia by flexing the joint and using a medial-lateral sawing motion on the posterior surface of the joint. If necessary, extend the joint to expose the anterior surface. Be careful to avoid cutting the bone.
- Once isolated, clean the bones of all adherent soft tissue. If performing four-point bending tests on the tibia, remove the fibula as well. The fibula is connected by ligaments at the proximal end but is fused to the tibia near the distal end of the bone. Use sharp scissors near the connection point to separate the fibula.
- Wrap the isolated and cleaned bones separately in saline-soaked gauze and store them at -20 °C. Do this immediately following harvest.
- Repeat steps 1.1.1 through 1.1.8 for the other side of the carcass.
NOTE: If there is resistance when trying to separate the bones in steps 1.1.4-1.1.6, it is best to repeat the steps rather than trying to pull the bones apart. Forceful movements may lead to damage or fracture of the bones.
- Thawing
NOTE: The number of freeze-thaw cycles a bone undergoes should be minimized as excessive freeze-thaw cycles can detrimentally impact the mechanical properties of bone. Partial thawing for µCT scanning may be achieved by leaving the bone at room temperature for 5-10 min. Only completely thaw the bone when performing bending tests as described below.- Overnight thaw-preferred
- Move the bones from -20 °C storage to 1-4°C in a cold room or refrigerator. Ensure the bones remain there for 8-12 h to fully thaw prior to testing.
- Quick-thaw
- Set the temperature of the bath to approximately 37 °C. Once at this temperature, add the bones to the bath.
- Leave the bones in the bath for approximately 1 h.
- Overnight thaw-preferred
2. µCT scanning
- Wrap the bones in parafilm prior to scanning to maintain hydration. Keep all other bones on ice while waiting to be scanned.
- Once wrapped in parafilm, place the bone into a holder to interface with the scanner. Ensure all scanned bones are aligned in the same orientation as consistent alignment will simplify rotation later in the analysis.
- Adjust the scan settings according to the application of the scan. The following general scan settings are recommended for mouse bones: resolution/voxel size: 10 µm; pixel size: medium, 2000 x 1048; filter: 0.5 mm aluminum; rotation step: 0.7; frame averaging: 2.
NOTE: These settings may differ depending on the system used to scan, and the manufacturer's and user manual should be consulted as needed. - Once the x-ray source is on, perform a flat field correction to minimize artifacts. To do this, first, ensure the chamber is empty and turn off the flat field.
- Measure the average intensity of the field and adjust it to 60%. Once at 60%, update the flat field and turn it back on.
- Ensure the average intensity is now (86-88%).
NOTE: This process may vary depending on the µCT system used. Consult the user manual prior to attempting the process. - Once the flat-field correction is successfully performed, place the holder in the chamber. Ensure the samples are centered and level before placing the pedestal in the chamber.
- Once the pedestal is secured, close the chamber, ensure the entire bone will be captured in the scan (a scout view may be necessary), and start the scan.
- Following scanning, re-store the bones in saline-soaked gauze at -20 °C.
3. µCT reconstruction
- Select an ROI that will capture the entire bone in reconstruction. To do this, view the largest cross section of bone and size the ROI based on this cross section.
- Set the thresholding of the software to allow for proper recognition of bone compared to non-bone. To do this, use a histogram in which a lower constraint is set at 0 and the upper constraint is set at the end of the peak histogram data.
- Adjust additional settings, including ring artifact reduction and beam hardening to 5 and 20%, respectively. Check that the misalignment compensation is within the range of -7 to 7. These values may vary according to the software. Ensure they are verified with the user manual and manufacturer-based instructions before beginning reconstruction.
NOTE: Artifacts can be minimized during reconstruction using corrections for beam hardening, ring artifacts, and misalignment compensation. Misalignment compensation may act as an indicator of the quality of the scan and if outside a manufacturer-specified range, the scan must be repeated. However, reconstruction settings will be software-dependent, and the user's manual should be consulted.
4. µCT rotation
NOTE: Once reconstructed, scans must be rotated to establish consistent orientation across all bones, and to ensure that transverse sections of the resulting bone are taken normal to the longitudinal axis with as little offset angle as possible. This should be done with the user's software of choice.
- Femur rotation
- Rotate the femur so all bones have the same longitudinal orientation. For example, orient all bones with the proximal end of the bone at the top of the scan.
- Rotate the bone so that the cross-sectional orientation of all bones is the same. For example, rotate the bones so that the anterior side is always on the right side of scans.
- Once these adjustments are made, straighten the scan to ensure symmetry is maintained about the central axis.
- Save the rotated dataset.
- Tibia rotation
- Repeat steps 4.1.1-4.1.4 for the tibia.
5. Mechanical testing procedure
- Preparation
- Prior to mechanical testing, ensure a 6-10 µm resolution µCT scan has been obtained and reconstructed to verify a quality scan has been acquired for each sample to calculate cross-sectional geometry at the fracture site (sections 2-3).
- With scans obtained and verified, thaw all bones prior to testing (section 1) . Test all bones from one experiment on the same day and randomize the order of testing to minimize user bias and system variability across samples and experimental groups. Ensure that the bones remain hydrated throughout the testing process.
- Apparatus setup
- Locate a load cell with appropriate sensitivity and capacity for the specimen. Consider the expected failure range for the specimen and choose a load cell with roughly 50% more capacity while maximizing sensitivity (e.g., a 10 lbf load cell with a 45 N capacity for a mouse bone in the 0-25 N failure range).
- Locate loading and support span fixtures.
- Install the load cell and fixtures as shown in Figure 1, by screwing the load cell onto either the top or bottom support of the tester, the top loading fixture onto the load cell, and the bottom fixture onto the bottom support of the tester. Ensure a secure fit.
NOTE: The attachment of the load cell to the top fixture is generally recommended when performing bending tests to avoid contact of fluid with the load cell but the bottom may be used if necessary. - Once the load cell and fixtures are installed, select a support span length and ensure it remains constant for all samples being tested. To choose a support span distance, first, locate the shortest bone in the sample set.
- Orient the bone between the fixtures as shown in Figure 2.
- For three-point bending of the femur, follow Figure 2A. Ensure the anterior surface of the bone is against the support span and the span region is within the diaphysis of the sample. Avoid including the third trochanter on the proximal end and the transition point where the bone widens into the metaphysis and condyles on the distal end.
- For four-point bending, ensure the support and loading spans are aligned and centered with one another. Follow Figure 2B to load the bone in the fixtures.
- Set the support and loading spans lengths to follow a 3:1 ratio8 (e.g., 9 mm support span and 3 mm loading span).
- For a tibia, load the medial surface of the bone against the support span with one support at the tibia/fibula junction. The other support will likely be positioned just after the tibial crest. Ensure the loading span, centered within the support span, then contains a uniform region of the bone.
- Measure the support span distance if performing three-point bending and both loading and support span distances if performing 4-point bending and record these distances. Ensure this value is recorded from the center of the loading points for both the loading and support span measurements.
- Place the bone back in saline or rehydrate with a bolus of saline.
NOTE: When selecting points for a loading span, it is recommended to use circular points (a 0.75 mm radius is sufficient as it distributes the load while also contacting the bone at the tangent of the circle). While theory recommends a knife edge to represent a point load, this will crush the bone at the point of load application, leading to overestimations of strain and underestimations of modulus. - Ensure all parts of the fixture are tight and free of movement.
- Software setup
- Ensure the tester is properly connected to the computer via the module box, load cell channels, and any other requirements per the system manual.
- In the software associated with the mechanical tester, create a bending test profile with a ramp that has a displacement rate that is slow enough to not induce viscoelastic effects (0.025 mm/s is often used) to load the bone to failure.
- A minimum sample frequency of 25 Hz is also recommended when creating a testing profile, although a higher sampling rate is preferred.
- Create one folder per study group and save each test as an individual file within that folder.
- Loading and testing samples
- Select a properly thawed bone (see step 1.2). Measure and record its full length with calipers.
- Load the sample onto the fixtures as shown in Figure 2A if testing a femur in three-point bending and Figure 2B if testing a tibia in four-point bending.
- Change the file name to reflect the sample being tested.
- Zero the load (not the displacement). Turn on the mover of the system; ensure it is not in load or displacement control.
- Using caution, apply a minimal preload to the bone to secure its position and help prevent the bone from rolling but ensure that it does not compromise the sample. Aim for a preload of approximately 0.25 N. Ensure the desired bone orientation is maintained before proceeding.
- Hydrate the sample by generously dousing it with saline.
- Begin the bending test by selecting Start or Run in the software. CRITICAL: Carefully watch the sample for the entirety of the test and note tests where any issues occurred (e.g., rolling, slipping).
NOTE: These issues could compromise data and notes about these tests will be helpful to consult during analysis. - Watch for the bone to begin to fracture (on the tensile side). Most tests will proceed until failure occurs. At this point, the test will terminate via its programmed limits. If failure occurs but the tester continues to displace, manually stop the test to prevent damage to the load cell.
- Once testing is complete, measure the length from the distal end to the break point using calipers and record it.
- Repeat steps 5.4.1 – 5.4.9 for each sample.
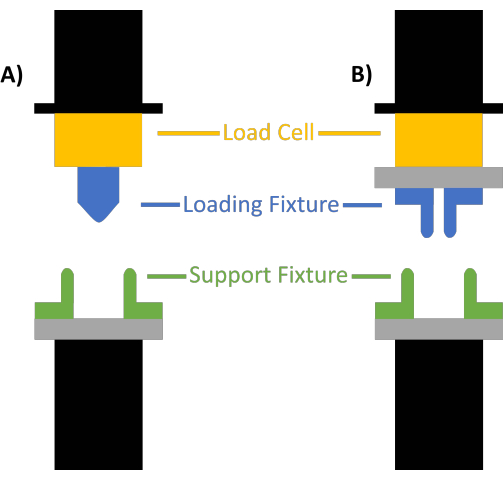
Figure 1: Mechanical tester setup. (A) Three-point and (B) four-point bending tests. The load cell is shown in yellow, the loading fixtures are shown in blue, and the support fixtures are shown in green. Please click here to view a larger version of this figure.

Figure 2: Orientation of bones between fixtures. (A) Proper orientation of a femur in three-point bending loading fixtures showing (from top to bottom) views from the medial, anterior, and posterior side of the femur when correctly positioned. The loading fixtures are shown in orange and the support fixtures are shown in blue. The bottom spans should be adjusted to include as much of the straightest part of the diaphysis as possible, and the top fixture should be centered between those spans. (B) Proper orientation of a tibia for four-point bending showing (from top to bottom) views from the anterior, lateral, and medial sides of the tibia. The bone should be loaded so that the medial surface contacts the bottom fixture, and the lateral surface contacts the top fixture. The tibia-fibula junction should be placed just outside of the loading span. The spans should be adjusted to best meet a loading-to-support span ratio of 1:3. Please click here to view a larger version of this figure.
6. ROI selection
- With the break lengths recorded, load the rotated images into the user's software of choice. Once the rotated images are loaded, locate and record the top and bottom slices of the bone.
- Calculate the difference between the top and bottom slices. Multiply this value by the scanning voxel size to determine the overall length of the bone in micrometers.
- To locate the fracture location in the CT scan, divide the recorded break length (in micrometers) by voxel size to obtain the number of µCT slices from the distal end of the scan to the break point.
- Select an ROI, centered at this location. First, set the total desired length of the ROI (at least 100 µm). Find the number of slices this length represents by dividing the length in micrometers by the voxel size to determine the total number of slices in the ROI.
- To obtain the lower bound of the ROI, divide the total number of ROI slices by 2 and subtract this value from the previously calculated break location found in step 6.4.
- Add the total length of the ROI in slices to the previously calculated value to obtain the upper bound of the ROI.
- Select the appropriate ROI, based on calculated bounds, and save it.
7. Normalization of force and displacement data
NOTE: The mechanical tester will only generate points with x and y coordinates (displacement, force). These points can be converted to stress and strain using the Euler-Bernoulli bending stress and strain equations, but these require geometric properties obtained from µCT scans. The quantification of these properties can be performed with the user's preferred software. We prefer a custom code, which gives complete control over all inputs, calculations, and outputs. As mentioned earlier, for access to the code, contact the corresponding author directly or visit the lab website at https://bbml.et.iupui.edu/ for more information. The stress and strain equations, as well as the necessary geometric properties that must be obtained from µCT scans, to calculate these are discussed below.
- Three-point bending normalization equations
- The equation used to calculate stress in three-point bending is shown below in Equation 1. In this equation, "F" represents force and "L" represents the length of the support span. Force values are recorded by the mechanical tester during the test. Ensure the length of the support span is recorded prior to testing. "c" and "I" are geometric properties that will be calculated using µCT scans (section 7.3).
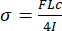 (1)
(1) - The equation to calculate strain is shown below in Equation 2; "c" and "L" represent the same properties for both stress and strain calculations. "d" signifies displacement values recorded by the mechanical tester during tests.
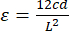 (2)
(2)
- The equation used to calculate stress in three-point bending is shown below in Equation 1. In this equation, "F" represents force and "L" represents the length of the support span. Force values are recorded by the mechanical tester during the test. Ensure the length of the support span is recorded prior to testing. "c" and "I" are geometric properties that will be calculated using µCT scans (section 7.3).
- Four-point bending normalization equations
- The equation for stress in four-point bending is shown below in Equation 3. "F" and "I" remain the same variables discussed in step 7.1.1. Calculate "a" from the measurements of the support and loading span prior to testing. If following the recommended ratio of 3:1 for support to loading span for four-point bending, "a" will be one-third the support span length.
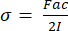 (3)
(3) - The equation for strain in four-point bending is shown below in Equation 4. "c" and "a" signify the same properties for both stress and strain calculations. "d" signifies the displacement values recorded by the mechanical tester during tests.
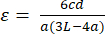 (4)
(4)
- The equation for stress in four-point bending is shown below in Equation 3. "F" and "I" remain the same variables discussed in step 7.1.1. Calculate "a" from the measurements of the support and loading span prior to testing. If following the recommended ratio of 3:1 for support to loading span for four-point bending, "a" will be one-third the support span length.
- Calculating geometrical properties from µCT scans
- The variable "c" represents the distance from the neutral axis to the surface of the bone that was loaded in tension. Consequently, determine the centroid of each cross section in the µCT scans since the neutral axis passes through the centroid.
- If following the testing orientation of a femur in three-point bending described in step 5.2.6, measure "c" with respect to the anterior surface.
- If following the testing orientation of a tibia described in step 5.2.7, measure "c" with respect to the medial surface of the bone.
- The variable "I" represents the area moment of inertia about the axis of bending (the medial-lateral axis for a femur; the anterior-posterior axis for a tibia). Calculate this value using Equation 5. In this equation, "dA" is the area of each pixel captured in the µCT scan while y is the calculated distance of each pixel from the neutral axis.
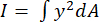 (5)
(5)
- The variable "c" represents the distance from the neutral axis to the surface of the bone that was loaded in tension. Consequently, determine the centroid of each cross section in the µCT scans since the neutral axis passes through the centroid.
8. Mechanical testing properties of interest
- Before calculating any mechanical properties, generate a force-displacement curve and stress-strain curve (ideal curves shown below in Figure 3, along with significant properties).
NOTE: Testing of biological samples does not always generate curves that look like these idealized examples, but they remain a useful guide. - Examine these curves prior to analysis to spot errors in testing, such as a bone rolling or slipping. These errors typically cause bumps or flat regions in the initial linear portion of the curve. Remove excess data, including any data that may have been collected prior to the tester contacting the bone or data following failure, at this point.
- Once assured of a quality test by the plotted curves, begin analysis of significant properties.
- Stiffness and elastic modulus
- Calculate stiffness using only the elastic region of the force-displacement curve. The slope of the curve in this region is stiffness.
- Calculate elastic modulus using the slope of only the elastic portion of the stress-strain curve.
- Yield point
NOTE: There are two yield points, one on the force-displacement curve and one on the stress-strain curve. The (x,y) values for this point from the force-displacement curve are known as displacement to yield and yield force, while those from the stress-strain curve are known as strain to yield and yield stress. These points represent the end of the elastic region of the curve and may be found in the ways listed below.- Stress-strain curve method: Calculate a line offset from (0,0) by 0.2% strain (2,000 microstrain) but with the same slope as the elastic modulus. Plot this line on the stress-strain graph; the position at which this line intercepts the stress-strain curve is defined as the yield point. Use this yield stress and strain coordinate to find the analogous force and displacement values; these values will represent yield force and displacement to yield values.
- Secant method: Calculate stiffness from the force-displacement curve and reduce the stiffness by a chosen percentage (5-10%). Plot a line starting at (0,0) with the slope of this reduced stiffness and allow it to intersect with the force-displacement curve. The point of intersection will have the coordinates (displacement to yield, yield force).
NOTE: The secant method may be used to find the yield point without stress-strain data.
- Ultimate force and ultimate stress
- Calculate ultimate force and ultimate stress by finding the maximum value in the respective data sets.
- Displacement and strain properties
- Displacement to yield and strain to yield values representing the displacement or strain to the yield point. To find them, locate the yield as described in step 8.3.2.
- Total displacement and total strain values represent the total displacement or total strain a sample experienced throughout the test and correspond to the failure point.
- Postyield displacement and postyield strain: Postyield displacement is commonly reported and may be calculated by subtracting displacement to yield from total displacement. Calculate postyield strain by subtracting strain to yield from total strain but report this with caution, as strain is first derived under the assumption that the material is linearly elastic (preyield). This makes a postyield measure susceptible to invalidity.
- Energy properties
- Calculate energy as the area under the force-displacement or the stress-strain curve.
- The area under the force-displacement curve is known as work. The area calculated under the pre-yield portion of the curve, or the elastic region, is known as elastic work or energy. The area calculated under the curve past the yield-point, or the plastic region, is known as post-yield or plastic work, or lost energy.
- The calculated total area under the stress-strain curve is known as toughness or modulus of toughness while the area calculated under the stress-strain curve up to the yield point is known as resilience. Post-yield toughness, like post-yield strain, is often not reported due to the assumptions of the strain equations that this property does not fall under.
- Stiffness and elastic modulus

Figure 3: Force-displacement and stress-strain curves. (A) Ideal force-displacement curve; (B) ideal stress-strain curve with the line derived from the 0.2% offset method used to calculate the yield point shown in red (note that this line has the same slope as that of the elastic region of the curve). Key properties that may be obtained from the force-displacement curve include yield force, ultimate force, displacement to yield, total displacement, and work. Tissue level properties that may be obtained from the stress-strain curve include yield stress, ultimate stress, strain to yield, total strain, resilience, and toughness. Please click here to view a larger version of this figure.
Results
Upon completion of CT scanning, most inadequate scans can be caught in reconstruction. Often, poor scans will have a high misalignment compensation that is a clear indicator of an error during the scan. However, errors may occur in other steps and could also lead to inaccurate data. These errors can often be spotted as the individual calculated architectural properties are examined. If values fall far out of the range of others in a group, the scan, ROI, and method of calculating the properties should be re-examined.
...Discussion
Throughout the scanning and testing process, there are moments when troubleshooting and optimization are appropriate. The first of these occurs when scanning bones using µCT. While many systems come with a holder in which one object may be held and scanned, custom holders can be fabricated to scan multiple bones at the same time. Scanning multiple bones can be an excellent point for optimization, but caution should be taken throughout the scanning and analysis process to ensure artifacts are not being induced. As X-...
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
The work done to develop this protocol has been supported by the National Institutes of Health [AR072609].
Materials
| Name | Company | Catalog Number | Comments |
| CTAn | Bruker | NA | CT Scan Analysis Software |
| DataViewer | Bruker | NA | CT Scan Rotation Software |
| Matrix Laboratory (MATLAB) 2023a | MathWorks | NA | Coding platform used for data analysis |
| NRecon | Bruker | NA | CT Scan Reconstruction software |
| SKYSCAN 1272-100 kV w/ 16 MP CCD detector, incl 3D Suite Software | Micro Photonics Inc | SKY-016814 | Micro-CT system that can non-destructively visualize up to 209 mPs in every virtual slice through an object |
References
- Vesper, E. O., Hammond, M. A., Allen, M. R., Wallace, J. M. Even with rehydration, preservation in ethanol influences the mechanical properties of bone and how bone responds to experimental manipulation. Bone. 97, 49-53 (2017).
- Jepsen, K. J., Silva, M. J., Vashishth, D., Guo, X. E., van der Meulen, M. C. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. Journal of Bone and Mineral Research. 30 (6), 951-966 (2015).
- Eds Burr, D. B., Allen, M. R. . Basic and Applied Bone Biology. , (2019).
- . . microCT SkyScan 1272 User Manual. , (2018).
- Kim, Y., Brodt, M. D., Tang, S. Y., Silva, M. J. MicroCT for scanning and analysis of mouse bones. Methods in Molecular Biology. 2230, 169-198 (2021).
- Bouxsein, M. L., et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of Bone and Mineral Research. 25 (7), 1468-1486 (2010).
- . . Micro-CT specimen scanner, Centre for high-throughput phenogenomics. , (2023).
- ASTM International. . Standard test method for flexural properties of unreinforced and reinforced plastics and electrical insulating materials by four-point bending. , (2020).
- ASTM International. . Standard test methods for flexural properties of unreinforced and reinforced plastics and electrical insulating materials. , (2017).
- . Bruker microCT NRecon: An overview. , (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved