A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Combining a Breath-Synchronized Olfactometer with Brain Simulation to Study the Impact of Odors on Corticospinal Excitability and Effective Connectivity
In This Article
Summary
This paper describes using a breath-synchronized olfactometer to trigger single- and dual-coil transcranial magnetic stimulation (TMS) during odorant presentation synchronized to human nasal breathing. This combination allows us to objectively investigate how pleasant and unpleasant odors impact corticospinal excitability and brain-effective connectivity in a given individual.
Abstract
It is widely accepted that olfactory stimulation elicits motor behaviors, such as approaching pleasant odorants and avoiding unpleasant ones, in animals and humans. Recently, studies using electroencephalography and transcranial magnetic stimulation (TMS) have demonstrated a strong link between processing in the olfactory system and activity in the motor cortex in humans. To better understand the interactions between the olfactory and the motor systems and to overcome some of the previous methodological limitations, we developed a new method combining an olfactometer that synchronizes the random order presentation of odorants with different hedonic values and the TMS (single- and dual-coil) triggering with nasal breathing phases. This method allows probing the modulations of corticospinal excitability and effective ipsilateral connectivity between the dorsolateral prefrontal cortex and the primary motor cortex that could occur during pleasant and unpleasant odor perception. The application of this method will allow for objectively discriminating the pleasantness value of an odorant in a given participant, indicating the biological impact of the odorant on brain effective connectivity and excitability. In addition, this could pave the way for clinical investigations in patients with neurological or neuropsychiatric disorders who may exhibit odor hedonic alterations and maladaptive approach-avoidance behaviors.
Introduction
It is widely accepted that olfactory stimulation elicits automatic reactions and motor behaviors. For example, in humans, the existence of an avoidance motor response (leaning away from the odor source) occurring 500 ms after negative odor onset has been recently demonstrated1. By recording freely moving human participants exploring odors emanating from flasks, Chalençon et al. (2022) showed that motor behaviors (i.e., speed of approach to the nose and withdrawal of the flask containing the odorant) are closely linked to odor hedonics2. Moreover, a close link between processing in the olfactory system and activity in the motor cortex has been recently demonstrated in humans by using electroencephalography1. Specifically, approximately 350 ms after the onset of negative odors, a specific mu rhythm desynchronization, known to reflect action preparation processes, was observed over and within the primary motor cortex (M1), shortly followed by a behavioral backward movement1. Strengthening the idea of a relationship between the olfactory and motor systems, another recent study showed that exposure to a pleasant odorant increased corticospinal excitability compared to a no-odor condition3. In this study, single-pulse transcranial magnetic stimulation (spTMS) was applied to M1 to evoke a motor-evoked potential (MEP) in a target hand muscle, recorded peripherally with electromyography (EMG) during odor perception. Exposure to the pleasant odorant was provided passively by paper strips sodden with pure bergamot essential oil and placed on a metal holder under the nose3. In this context, it remains unclear whether the facilitation of the corticospinal excitability is due to the pleasant odorant stimulation or to unspecific behavioral effects such as sniffing and teeth clenching4,5. Furthermore, it is still unknown how an unpleasant odorant modulates M1 excitability probed by TMS.
In summary, this highlights the need to develop a method that offers the following advantages over existing techniques used in previous studies3,6: (1) randomizing the presentation of different odor conditions (pleasant/unpleasant/no-odor) within the same experimental phase, (2) precisely synchronizing odorant presentation and TMS timing according to the human nasal breathing phases (inspiration and expiration) when studying the motor system.
TMS can also be used as a tool to investigate cortico-cortical interactions, also called effective connectivity, between multiple cortical areas and M1 with a high temporal resolution7,8,9,10,11,12. Here, we use a dual-site TMS (dsTMS) paradigm, in which a first-conditioning stimulation (CS) activates a target cortical area, and a second-test stimulation (TS) is applied over M1 using another coil to evoke an MEP. The effect of the CS is evaluated by normalizing the amplitude of the conditioned MEP (dsTMS condition) to the amplitude of the unconditioned MEP (spTMS condition)13. Then, negative ratio values indicate suppressive cortico-cortical interactions, while positive ratio values indicate facilitatory cortico-cortical interactions between the two stimulated areas. The dsTMS paradigm thus provides a unique opportunity to identify the nature (i.e., facilitatory or suppressive), the strength, and the modulations of the effective connectivity between the preactivated area and M1. Importantly, cortico-cortical interactions reflect a complex balance of facilitation and suppression that may be modulated in different timing and mental states or tasks7,14.
To our knowledge, the relatively new dsTMS paradigm has never been used to investigate cortico-cortical interactions during odor perception with different hedonic values. However, neuroimaging studies have shown that exposure to pleasant and unpleasant odorants induces connectivity changes in areas involved in emotion, decision-making, and action control, including the supplementary motor area, the anterior cingulate cortex, and the dorsolateral prefrontal cortex (DLPFC)15,16. Indeed, the DLPFC is a key node mediating emotional control, sensory processing, and higher-level aspects of motor control, such as preparatory processes17,18,19. In addition, both human and animal studies have provided evidence that the DLPFC has diverse neuronal projections to M117,18,20,21,22. Depending on the context, these DLPFC projections can either facilitate or inhibit M1 activity7,19,20. Thus, it seems possible that the effective connectivity between DLPFC and M1 is modulated during odor presentation and that pleasant and unpleasant odorants recruit separated cortical networks, leading to a differential effect on DLPFC-M1 connectivity.
Here, we propose a new method suitable for the methodologically rigorous study of the modulations of corticospinal excitability and effective connectivity that might occur during the perception of pleasant and unpleasant odors, all delivered in synchrony with human nasal breathing.
Protocol
All experimental procedures described in the following sections have been approved by an Ethics Committee (CPP Ile de France VII, Paris, France, protocol number 2022-A01967-36) in accordance with the Declaration of Helsinki. All participants provided written informed consent before study enrollment.
1. Participant recruitment
- Inclusion/exclusion criteria.
- Include adult (> 18 years) participants. Screen all participants for any contraindications to TMS according to international expert guidelines23.
- Exclude participants with implanted medical devices (e.g., cochlear implant, cardiac pacemaker, etc.), a personal or family history of seizure, headache, brain trauma, and neuroactive medication. Exclude participants considered "anosmic" according to the European Test of Olfactory Capabilities24.
- Handedness: Check for right-handedness as assessed by the Edinburgh Handedness Inventory questionnaire25.
NOTE. It is highly recommended to recruit only right-handed participants in studies assessing corticospinal excitability and effective connectivity in the motor system26,27. - Information and informed consent: Give all participants basic information about the study objectives, procedures, and risks approved by the Ethics Committee and ask them to sign written informed consent.
2. Experimental procedure
- Patient installation: Ask the participant to sit on a comfortable chair (dental chair type) with both hands relaxed and pronated. Position the paricipant's head on a chin rest to minimize head movement during stimulation.
- Electromyography recordings
- Prepare the participant's skin before electrode application using an exfoliant scrub to lightly abrade the areas and clean the areas using alcohol pads where electrodes will be applied.
- Apply two silver/silver chloride disposable recording electrodes with a belly-tendon montage of the first dorsal interosseous (FDI) muscle. Add the ground electrode to the styloid process of the ulna (Figure 1).
- Connect the electrodes to the amplifier with cables and the data acquisition system.
- Record the EMG signal using an analog-to-digital (AD) conversion system. Amplify and filter EMG signals (gain = 1000) using a bandwidth frequency between 10 Hz and 1 kHz. Digitize at a sampling rate of 2,000 Hz and store each EMG file for offline analysis.
- Check the quality of the signal displayed on the computer screen connected to the data acquisition system.
- TMS coilM1 position.
- Connect this coil to the A stimulator (Figure 1).
- Place a tight-fitting cap over the participant's head. Use a tape measure to perform nasion-inion, tragus-tragus, and head-circumference measurements based on standard cranial landmarks. Identify and mark with a pen the scalp vertex at the intersection of the mid-sagittal (nasion-inion) and interaural (tragus-tragus) lines28.
- Place tangentially to the scalp the first small figure-of-eight coil (inner diameter: 40 mm) over the presumed hand area of the left M1 (coilM1), which is 5 cm lateral from the vertex, with the handle pointing backward and laterally at a 45° angle to the midsagittal line, resulting in a posterior-anterior current flow (monophasic current waveform). This orientation corresponds to a maximum induced current flowing within M1 within M129.
- Ensure that the placement of the coilM1 is optimal, in accordance with the most recent international recommendations30. Start by delivering a few single pulses at 30% of the maximum stimulator output (%MSO) and check that the stimulation produces an MEP as recorded by the EMG system and displayed on the computer screen connected to the data acquisition system.
- If there are no visible responses, gradually increase the stimulation intensity (5 %MSO increments) until MEPs are observed. Then, test four spots around the first site by delivering multiple pulses. Determine the mean peak-to-peak MEP amplitude for each site.
- Select the location where the average peak-to-peak MEP amplitude is the highest. This is the so-called hotspot location for the participant30. Mark the coilM1 location on the cap to ensure proper coil placement throughout the experiment.
- Resting motor threshold (rMT) and TMS intensities
- Determine the resting motor threshold (rMT) defined as the TMS intensity that produces a 50% probability of eliciting an MEP23,30.
- Use the available online freeware (TMS Motor Threshold Assessment Tool, MTAT 2.1), which is based on a maximum-likelihood parameter estimation using a sequential testing strategy29. The stimulation sequence always starts with the intensity set at 37 %MSO.
- Let one experimenter hold the coilM1 while another indicates whether the MEP amplitude is > 0.05 mV. The predictive algorithm then determines the next stimulation intensity to be delivered and is stopped after 20 stimulations, which provides sufficient accuracy for the rMT estimation according to previous studies31-34.
- Set the %MSO for the conditioning and the test pulse stimulation. Use the previously determined rMT value of the participant.
NOTE: Here, the intensity for the first conditioning stimulation (coilDLPFC) was set to 110% of the rMT19,20. The intensity of the test stimulation (coilM1) was set at 120% of the rMT, an intensity that differs slightly from previous studies that used a TS intensity that evoked a MEP of ~1 mV in all participants19,20 . This fixed peak-to-peak intensity occurs at very different points on the input-output recruitment curves due to the high inter-subject variability in motor output35. Therefore, the stimulation intensity could be optimized using 120% RMT intensity across individuals.
- Determine the resting motor threshold (rMT) defined as the TMS intensity that produces a 50% probability of eliciting an MEP23,30.
- TMS coilDLPFC positioning
- Connect this coil to the B stimulator (Figure 1).
- Use the recently updated scalp heuristic to locate the region of the scalp corresponding to the left DLPFC36,37 to estimate the position of the second small figure-of-eight coil (internal diameter: 40 mm) over the DLPFC (coilDLPFC). Download the online Excel Spreadsheet Calculation Tool36 and enter the nasion-inion and tragus-tragus distances and the head circumference in centimeters as inputs. Report the XLA and the YLA distances directly on the participant's head.
- Place tangentially to the scalp the coilDLPFC over the presumed left DLPFC location, with the handle pointing downward and laterally at a -45° angle to the mid-sagittal line. Mark the coilDLPFC placement on the cap to ensure proper coil placement throughout the experiment.
NOTE: This scalp-based targeting method for both coilM1 and coilDLPFC locations is not optimal. In fact, it is known to be less accurate than the neuronavigation method used to target the brain areas of interest based on individual T1 anatomical magnetic resonance imaging (MRI)38.
- Delay between the conditioning and test pulses: Set this delay to 10 ms on the pulse generator device.
NOTE: Here, the delay is fixed at 10 ms based on previous studies showing an inhibitory influence from the left DLPFC to the left M1 at this interval19,20. This inhibitory effect observed at 10 ms is likely due to the activation of the basal ganglia via the DLPFC projections to the pre-SMA, thereby exerting an indirect influence on M139. The delay can be adjusted in the code according to the user's needs. For example, a longer interstimulation interval (i.e., 25 ms) could be used to investigate polysynaptic indirect cortico-subcortical-cortical circuits connecting DLPFC to M119. Furthermore, differential facilitatory/inhibitory influences have been demonstrated using dual-site ppTMS between multiple cortical areas, with intervals ranging from 1 ms to 150 ms40,41. Thus, the fact that the interval can be adjusted opens the way to a wide range of possibilities for future research studies. - Olfactometer settings
- Select odorants with pleasant and unpleasant hedonic values. Dilute in advance the odorants individually in mineral oil to create iso-intense perception.
NOTE: Here, the selection and concentration of odorants (i.e., isoamyl acetate and butyric acid diluted to 0.6% and 0.11% vol/vol concentrations, respectively) were based on previous studies by our group using the same olfactometer setup and odorants42,43. A pilot study confirms that the positive and the negative odors did not differ in terms of intensity but were opposite in hedonic value. In the control condition (i.e., no odorant), only airflow is delivered to the participant. - Write the code to deliver the odorants. For each trial, indicate the total duration of the trial, the odorant to be delivered, the flow rate of the odorant controller (in milliliters per minute), the flow rate of the carrier air regulator (in milliliters per minute ), and the flow rate of the suction regulator.
NOTE: The order of the odorant delivered can be randomized between positive, negative, and no-odor. Here, each trial has a duration of 12 s. The order of odor delivered was pseudo-randomized. In addition, based on a pilot experiment, the flow rate of the odor controller was set to 200 mL/min, the flow rate of the carrier air regulator at 500 mL/min, and the flow rate of the suction regulator at 100 mL/min. - Position the nasal cannula near the participant's nostrils to measure nasal breathing. Instruct the participant to breathe normally through the nose.
- Turn on the portable air compressor, the olfactometer case, and the PC containing the software. Check all the cable connections (Figure 1).
NOTE: The olfactometer used in the current study has been described in detail in a previous publication44 but has been modified here to allow TMS triggering with variable delays after inspiration onset detection.Briefly, the device is composed of several modules, including 1) an air source and air treatment system coming from a portable air compressor, 2) a stimulation system including electronic and pneumatic devices, 3) a homemade mixing head coupled to a delivery system that allows the diffusion of odorants in the participant's nose, 4) a respiratory sensory system that triggers the olfactometer according to the nasal respiration measurement with a nasal cannula and 5) a software control system44. - Calibration: Proceed to the calibration phase (about 20 s.), which allows for calibrating the participant's respiratory signal and adjusting the detection thresholds of the expiratory and inspiratory phases. In this software, the expiratory phase is positive, and the inspiratory phase is negative.
- Odor hedonic and intensity ratings: Deliver the two odorants in a randomized order and ask participants to rate the hedonic value and intensity of each odorant on visual analog scales ranging from 1 "not at all pleasant" to 9 "extremely pleasant" and from 1 "not at all intense" to "extremely intense."
- Select odorants with pleasant and unpleasant hedonic values. Dilute in advance the odorants individually in mineral oil to create iso-intense perception.
- Combining olfactometer and TMS: Set the delay between the detection of the inspiration phase and the trigger for sending the TMS at 600 ms.
NOTE: The setting of the delay is important and has to be determined according to the literature and the needs of the user. In this protocol, the delay was set at 600 ms, which has been shown to be the maximum conscious perceptual representation of odors45. For single-pulse TMS condition, this trigger immediately activates the A stimulator, and a pulse is delivered by the coil positioned on the left M1 to evoke an unconditioned MEP.For dual-coil TMS condition, this trigger is sent to two different devices (via two coaxial cables connected by a T-connection): the first one immediately activates the B stimulator and a conditioning pulse is delivered by the coil positioned on the left DLPFC; the second one is received by a pulse generator which makes it possible to induce a fixed delay before activating the A stimulator, thus delivering a test-stimulation through the coil positioned on the left M1 to evoke a conditioned MEP (Figure 1).
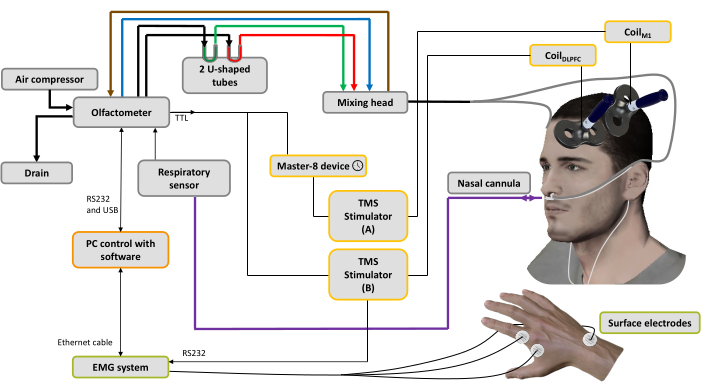
Figure 1: Experimental setup. The bold lines represent pneumatic connections. An air compressor is connected to the olfactometer to generate different air flows. A regulator controls the pressure, and the input air flow is directed to 3 channels (through 3 mass regulators): one for the air conveyor (blue line), one for the aspiration system (brown line) to clean and help control the stimulation time and the last one for the odorants44. Two U-shaped tubes contain the odorants (green: pleasant; red: unpleasant) in which they are conditioned under pressure in the saturated steam state, ensuring an odorized air flow with stable intensity over time. The mixing head is used to mix the clean and odorized air streams. The airflow (odorized or pure) is delivered to the nostrils through two tubes (gray lines) attached to a nasal cannula, which is also used to record nasal breathing (purple line). Based on the respiratory signal, as soon as the inhalation phase is detected, for the spTMS condition a trigger is sent to a pulse generator device used to set a delay (here: 10 ms), then to a TMS stimulator A connected to CoilM1 applied over the left M1 hand muscle representation, while the TMS stimulator B is turned off. For the dsTMS condition, a trigger is immediately sent to the TMS stimulator B connected to the CoilDLPFC applied over the left DLPFC, and the pulse generator device is used to set a delay (here: 10 ms) before triggering the TMS stimulator A connected to the CoilM1. The respiratory signal and MEP amplitude acquired by the EMG system are recorded by software installed on a PC. Please click here to view a larger version of this figure.
3. Measurements
- Run the custom-made coding script in the olfactometer software (see step 2.7.2) to deliver all combinations of spTMS and dsTMS with pleasant and unpleasant odors and no-odors occurring in a random order.
NOTE: Here, 20 trials were recorded for each condition (120 trials in total). The experiment was divided into 6 blocks of 20 trials each. The number of trials for each condition can be changed according to the user's needs.
4. Data analyses
- For each participant, condition, and trial, extract the peak-to-peak MEP amplitude. This can be done using one of the open-source Toolboxes available online46,47.
- Normalize the data by calculating an MEP ratio expressing MEPs elicited by the test stimulation in dsTMS trials relative to MEPs elicited by the test stimulation in spTMS trials12. Do this separately for each participant and for each odor condition (i.e., no-odor, positive odor, and negative odor). After this procedure, interpret the results as follows: MEP ratios above 1 indicate a facilitatory influence of the DLPFC on M1, whereas MEP ratios below 1 indicate an inhibitory influence of the DLPFC on M1.
Results
The representative data presented here reflect recordings from participants after completing the step-by-step protocol above to provide a preliminary insight into what we might expect.
Figure 2 shows an example of a representative participant's respiratory signals recorded with the olfactometer software. The expiratory and inspiratory phases are well detected when the thresholds are crossed. The odorant is triggered immediately after the expiration phase ...
Discussion
The protocol above describes a novel method combining the use of a breath-synchronized olfactometer with single- and dual-coil TMS to investigate changes in corticospinal excitability and effective connectivity depending on the hedonic value of the odorants. This setup will allow for objectively discriminating the pleasantness value of an odorant in a given participant, indicating the biological impact of the odorant on brain effective connectivity and reactivity. The critical steps in this protocol involve both TMS...
Disclosures
JB is a board member of the Brain Stimulation Section (STEP) of the French Association of Biological Psychiatry and Neuropsychopharmacology (AFPBN), of the European Society of Brain Stimulation (ESBS), and reports academic research grants in the field of brain stimulation from CIHR (Canada), ANR and PHRC (France). Other authors have nothing to disclose.
Acknowledgements
This work was supported by the Fondation de France, Grant N°: 00123049/WB-2021-35902 (a grant received by J.B. and N.M.). The authors would like to thank the Fondation Pierre Deniker for its support (grant received by C.N.) and the staff of the Neuro-Immersion platform for their valuable help in designing the setup.
Materials
| Name | Company | Catalog Number | Comments |
| Acquisition board (8 channels) | National Instrument | NI USB-6009 | |
| Air compressor | Jun-Air | Model6-15 | |
| Alcohol prep pads | Any | ||
| Butyric acid | Sigma-Aldrich | B103500 | Negative odorant |
| Desktop computer | Dell | Latitude 3520 | |
| EMG system | Biopac System | MP150 | |
| Isoamyl acetate | Sigma-Aldrich | W205508 | Positive odorant |
| Nasal cannula | SEBAC France | O1320 | |
| Programmable pulse generator | A.M.P.I | Master-8 | |
| Surface electrodes | Kendall Medi-trace | FS327 | |
| TMS coil (X2) | MagStim | D40 Alpha B.I. coil | |
| TMS machine | MagStim | Bistim2 | |
| Tube 6 mm x 20 m | Radiospare | 686-2671 | Pneumatic connection |
| USB-RS232 | Radiospare | 687-7806 | |
| U-shaped tubes | VS technologies | VS110115 |
References
- Iravani, B., Schaefer, M., Wilson, D. A., Arshamian, A., Lundström, J. N. The human olfactory bulb processes odor valence representation and cues motor avoidance behavior. Proceedings of the National Academy of Sciences. 118 (42), e2101209118 (2021).
- Chalençon, L., Thevenet, M., Noury, N., Bensafi, M., Mandairon, N. Identification of new behavioral parameters to assess odorant hedonic value in humans: A naturalistic approach. Journal of Neuroscience Methods. 366, 109422 (2022).
- Infortuna, C., et al. Motor cortex response to pleasant odor perception and imagery: The differential role of personality dimensions and imagery ability. Frontiers in Human Neuroscience. 16, 943469 (2022).
- Ozaki, I., Kurata, K. The effects of voluntary control of respiration on the excitability of the primary motor hand area, evaluated by end-tidal CO2 monitoring. Clinical Neurophysiology. 126 (11), 2162-2169 (2015).
- Boroojerdi, B., Battaglia, F., Muellbacher, W., Cohen, L. G. Voluntary teeth clenching facilitates human motor system excitability. Clinical Neurophysiology. 111 (6), 988-993 (2000).
- Rossi, S., et al. Distinct olfactory cross-modal effects on the human motor system. PLOS One. 3 (2), e1702 (2008).
- Neige, C., Rannaud Monany, D., Lebon, F. Exploring cortico-cortical interactions during action preparation by means of dual-coil transcranial magnetic stimulation: A systematic review. Neuroscience and Biobehavioral Reviews. 128 (October 2020), 678-692 (2020).
- Koch, G. Cortico-cortical connectivity: the road from basic neurophysiological interactions to therapeutic applications. Experimental Brain Research. 238 (7-8), 1677-1684 (2020).
- Derosiere, G., Vassiliadis, P., Duque, J. Advanced TMS approaches to probe corticospinal excitability during action preparation. NeuroImage. 213 (November 2019), 116746 (2020).
- Goldenkoff, E. R., Mashni, A., Michon, K. J., Lavis, H., Vesia, M. Measuring and manipulating functionally specific neural pathways in the human motor system with transcranial magnetic stimulation. Journal of Visualized Experiments JoVE. 156, 60706 (2020).
- Malderen, S. V., Hehl, M., Verstraelen, S., Swinnen, S. P., Cuypers, K. Dual-site TMS as a tool to probe effective interactions within the motor network: a review. Reviews in the Neurosciences. 34 (2), 129-221 (2023).
- Neige, C., et al. Connecting the dots: Harnessing dual-site transcranial magnetic stimulation to assess the causal influence of medial frontal areas on the motor cortex. Cerebral Cortex. , bhad370 (2023).
- Ferbert, A., Priori, A., Rothwell, J. C., Day, B. L., Colebatch, J. G., Marsden, C. D. Interhemispheric inhibition of the human motor cortex. The Journal of physiology. 453, 525-546 (1992).
- Rothwell, J. C. Using transcranial magnetic stimulation methods to probe connectivity between motor areas of the brain. Human Movement Science. 30 (5), 906-915 (2011).
- Carlson, H., Leitão, J., Delplanque, S., Cayeux, I., Sander, D., Vuilleumier, P. Sustained effects of pleasant and unpleasant smells on resting state brain activity. Cortex. 132, 386-403 (2020).
- Farruggia, M. C., Pellegrino, R., Scheinost, D. Functional connectivity of the chemosenses: A review. Frontiers in Systems Neuroscience. 16, 865929 (2022).
- Hasan, A., Galea, J. M., Casula, E. P., Falkai, P., Bestmann, S., Rothwell, J. C. Muscle and timing-specific functional connectivity between the dorsolateral prefrontal cortex and the primary motor cortex. Journal of Cognitive Neuroscience. 25 (4), 558-570 (2013).
- Brown, M. J. N., Goldenkoff, E. R., Chen, R., Gunraj, C., Vesia, M. Using dual-site transcranial magnetic stimulation to probe connectivity between the dorsolateral prefrontal cortex and ipsilateral primary motor cortex in humans. Brain Sciences. 9 (8), 177 (2019).
- Xia, X., et al. Connectivity from ipsilateral and contralateral dorsolateral prefrontal cortex to the active primary motor cortex during approaching-avoiding behavior. Cortex. 157, 155-166 (2022).
- Wang, Y., Cao, N., Lin, Y., Chen, R., Zhang, J. Hemispheric differences in functional interactions between the dorsal lateral prefrontal cortex and ipsilateral motor cortex. Frontiers in Human Neuroscience. 14, 1-6 (2020).
- Gabbott, P. L. A., Warner, T. A., Jays, P. R. L., Salway, P., Busby, S. J. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology. 492 (2), 145-177 (2005).
- Yeterian, E. H., Pandya, D. N., Tomaiuolo, F., Petrides, M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 48 (1), 58-81 (2012).
- Rossi, S., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert guidelines. Clinical Neurophysiology. 132 (1), 269-306 (2021).
- Joussain, P., et al. Application of the European Test of Olfactory Capabilities in patients with olfactory impairment. European Archives of Oto-Rhino-Laryngology. 273 (2), 381-390 (2016).
- Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 9 (1), 97-113 (1971).
- Daligadu, J., Haavik, H., Yielder, P. C., Baarbe, J., Murphy, B. Alterations in cortical and cerebellar motor processing in subclinical neck pain patients following spinal manipulation. Journal of Manipulative and Physiological Therapeutics. 36 (8), 527-537 (2013).
- Andersen, K. W., Siebner, H. R. Mapping dexterity and handedness: recent insights and future challenges. Current Opinion in Behavioral Sciences. 20, 123-129 (2018).
- Fried, P. J., et al. Training in the practice of noninvasive brain stimulation: Recommendations from an IFCN committee. Clinical Neurophysiology. 132 (3), 819-837 (2021).
- Mills, K. R., Boniface, S. J., Schubert, M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalography and Clinical Neurophysiology. 85 (1), 17-21 (1992).
- Rossini, P. M., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology. 126 (6), 1071-1107 (2015).
- Awiszus, F. TMS and threshold hunting. Supplements to Clinical Neurophysiology. 56, 13-23 (2003).
- Awiszus, F. Using relative frequency estimation of transcranial magnetic stimulation motor threshold does not allow to draw any conclusions about true threshold. Clinical Neurophysiology. 125 (6), 1285-1286 (2014).
- Ah Sen, C. B., Fassett, H. J., El-Sayes, J., Turco, C. V., Hameer, M. M., Nelson, A. J. Active and resting motor threshold are efficiently obtained with adaptive threshold hunting. PLoS One. 12 (10), 1-9 (2017).
- Neige, C., Rannaud Monany, D., Stinear, C. M., Byblow, W. D., Papaxanthis, C., Lebon, F. Unravelling the modulation of intracortical inhibition during motor imagery: An adaptive threshold-hunting study. Neuroscience. 434, 102-110 (2020).
- Burke, D., Pierrot-Deseilligny, E. Caveats when studying motor cortex excitability and the cortical control of movement using transcranial magnetic stimulation. Clinical Neurophysiology. 121 (2), 121-123 (2010).
- Mir-Moghtadaei, A., et al. Updated scalp heuristics for localizing the dorsolateral prefrontal cortex based on convergent evidence of lesion and brain stimulation studies in depression. Brain Stimulation. 15 (2), 291-295 (2022).
- Siddiqi, S. H., et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nature Human Behaviour. 5 (12), 1707-1716 (2021).
- Caulfield, K. A., Fleischmann, H. H., Cox, C. E., Wolf, J. P., George, M. S., McTeague, L. M. Neuronavigation maximizes accuracy and precision in TMS positioning: Evidence from 11,230 distance, angle, and electric field modeling measurements. Brain Stimulation. 15 (5), 1192-1205 (2022).
- Cao, N., et al. Plasticity changes in dorsolateral prefrontal cortex associated with procedural sequence learning are hemisphere-specific. NeuroImage. 259, 119406 (2022).
- Brown, M. J. N., et al. Somatosensory-motor cortex interactions measured using dual-site transcranial magnetic stimulation. Brain Stimulation. 12 (5), 1229-1243 (2019).
- Fiori, F., Chiappini, E., Candidi, M., Romei, V., Borgomaneri, S., Avenanti, A. Long-latency interhemispheric interactions between motor-related areas and the primary motor cortex: a dual site TMS study. Scientific reports. 7 (1), 14936 (2017).
- Fournel, A., Ferdenzi, C., Sezille, C., Rouby, C., Bensafi, M. Multidimensional representation of odors in the human olfactory cortex. Human Brain Mapping. 37 (6), 2161-2172 (2016).
- Midroit, M., et al. Neural processing of the reward value of pleasant odorants. Current Biology. 31 (8), 1592-1605.e9 (2021).
- Sezille, C., Messaoudi, B., Bertrand, A., Joussain, P., Thévenet, M., Bensafi, M. A portable experimental apparatus for human olfactory fMRI experiments. Journal of Neuroscience Methods. 218 (1), 29-38 (2013).
- Kato, M., et al. Spatiotemporal dynamics of odor representations in the human brain revealed by EEG decoding. Proceedings of the National Academy of Sciences. 119 (21), e2114966119 (2022).
- Jackson, N., Greenhouse, I. VETA: An open-source Matlab-based toolbox for the collection and analysis of electromyography combined with transcranial magnetic stimulation. Frontiers in Neuroscience. 13, 975 (2019).
- Cunningham, D., Zhang, B., Cahn, A. Transcranial magnetic stimulation (TMS) analysis toolbox: A user friendly open source software for basic and advanced analysis and data sharing of TMS related outcomes. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 14 (6), 1641-1642 (2021).
- Julkunen, P., Säisänen, L., Hukkanen, T., Danner, N., Könönen, M. Does second-scale intertrial interval affect motor evoked potentials induced by single-pulse transcranial magnetic stimulation. Brain Stimulation. 5 (4), 526-532 (2012).
- Pellicciari, M. C., Miniussi, C., Ferrari, C., Koch, G., Bortoletto, M. Ongoing cumulative effects of single tms pulses on corticospinal excitability: An intra- and inter-block investigation. Clinical Neurophysiology. 127 (1), 621-628 (2016).
- Li, S., Rymer, W. Z. Voluntary breathing influences corticospinal excitability of nonrespiratory finger muscles. Journal of Neurophysiology. 105 (2), 512-521 (2011).
- Boesveldt, S., Frasnelli, J., Gordon, A. R., Lundström, J. N. The fish is bad: Negative food odors elicit faster and more accurate reactions than other odors. Biological Psychology. 84 (2), 313-317 (2010).
- Neige, C., Mavromatis, N., Gagné, M., Bouyer, L. J., Mercier, C. Effect of movement-related pain on behaviour and corticospinal excitability changes associated with arm movement preparation. Journal of Physiology. 596 (14), 2917-2929 (2018).
- Bergmann, T. O., Hartwigsen, G. Inferring causality from noninvasive brain stimulation in cognitive neuroscience. Journal of Cognitive Neuroscience. 33 (2), 195-225 (2021).
- Kulason, S., et al. A comparative neuroimaging perspective of olfaction and higher-order olfactory processing: on health and disease. Seminars in Cell & Developmental Biology. 129, 22-30 (2022).
- Athanassi, A., Dorado Doncel, R., Bath, K. G., Mandairon, N. Relationship between depression and olfactory sensory function: a review. Chemical Senses. 46, bjab044 (2021).
- Grimm, S., et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fmri study in severe major depressive disorder. Biological Psychiatry. 63 (4), 369-376 (2008).
- Naudin, M., El-Hage, W., Gomes, M., Gaillard, P., Belzung, C., Atanasova, B. State and trait olfactory markers of major depression. PLOS One. 7 (10), e46938 (2012).
- Guidali, G., Roncoroni, C., Bolognini, N. Modulating frontal networks' timing-dependent-like plasticity with paired associative stimulation protocols: Recent advances and future perspectives. Frontiers in Human Neuroscience. 15, 658723 (2021).
- Hernandez-Pavon, J. C., San Agustín, A., Wang, M. C., Veniero, D., Pons, J. L. Can we manipulate brain connectivity? A systematic review of cortico-cortical paired associative stimulation effects. Clinical Neurophysiology. 154, 169-193 (2023).
- Deng, Z. -. D., Robins, P. L., Dannhauer, M., Haugen, L. M., Port, J. D., Croarkin, P. E. Optimizing TMS coil placement approaches for targeting the dorsolateral prefrontal cortex in depressed adolescents: An electric field modeling study. Biomedicines. 11 (8), 2320 (2023).
- Gomez, L. J., Dannhauer, M., Peterchev, A. V. Fast computational optimization of TMS coil placement for individualized electric field targeting. NeuroImage. 228, 117696 (2021).
- Derosiere, G., Duque, J. Tuning the corticospinal system: How distributed brain circuits shape human actions. The Neuroscientist. 26 (4), 359-379 (2020).
- Bestmann, S., Krakauer, J. W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Experimental Brain Research. 233 (3), 679-689 (2015).
- Reis, J., et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. Journal of Physiology. 586 (2), 325-351 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved