A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Troubleshooting and Quality Assurance in Hyperpolarized Xenon Magnetic Resonance Imaging: Tools for High-Quality Image Acquisition
In This Article
Summary
Here, we present a protocol for obtaining high-quality hyperpolarized xenon-129 magnetic resonance images, covering hardware, software, data acquisition, sequence selection, data management, k-space utilization, and noise analysis.
Abstract
Hyperpolarized (HP) xenon magnetic resonance imaging (129Xe MRI) is a recently federal drug administration (FDA)-approved imaging modality that produces high-resolution images of an inhaled breath of xenon gas for investigation of lung function. However, implementing 129Xe MRI is uniquely challenging as it requires specialized hardware and equipment for hyperpolarization, procurement of xenon imaging coils and coil software, development and compilation of multinuclear MR imaging sequences, and reconstruction/analysis of acquired data. Without proper expertise, these tasks can be daunting, and failure to acquire high-quality images can be frustrating, and expensive. Here, we present some quality control (QC) protocols, troubleshooting practices, and helpful tools for129Xe MRI sites, which may aid in the acquisition of optimized, high-quality data and accurate results. The discussion will begin with an overview of the process for implementing HP 129Xe MRI, including requirements for a hyperpolarizer lab, the combination of 129Xe MRI coil hardware/software, data acquisition and sequence considerations, data structures, k-space and image properties, and measured signal and noise characteristics. Within each of these necessary steps lies opportunities for errors, challenges, and unfavorable occurrences leading to poor image quality or failed imaging, and this presentation aims to address some of the more commonly encountered issues. In particular, identification and characterization of anomalous noise patterns in acquired data are necessary to avoid image artifacts and low-quality images; examples will be given, and mitigation strategies will be discussed. We aim to make the 129Xe MRI implementation process easier for new sites while providing some guidelines and strategies for real-time troubleshooting.
Introduction
For over a century, lung function assessment has primarily relied on global measurements from spirometry and body plethysmography. However, these traditional pulmonary function tests (PFTs) are limited in their ability to capture early-stage disease's regional nuances and subtle changes in lung tissue1. Nuclear medicine with inhaled radiotracers has been used widely for the assessment of ventilation/perfusion mismatches commonly associated with pulmonary emboli, but this involves ionizing radiation and yields lower resolution. In contrast, computed tomography (CT) has emerged as the gold standard for lung imaging, offering exceptional spatial and temporal clarity compared to nuclear imaging2. While low-dose CT scans can mitigate radiation exposure, potential radiation risk should still be considered3,4. Proton MRI of the lung is uncommon due to low tissue density of the lung and rapid signal decay from lung tissue, although recent advances offer functional information despite potential low signal. On the other hand, hyperpolarized xenon magnetic resonance imaging (HP 129Xe MRI) is a non-invasive modality that allows for imaging of lung function with regional specificity5,6. It produces a high nonequilibrium nuclear magnetization of the gas in liter quantities. The inert gas is then inhaled by a subject inside the MR scanner for a single breath and is directly imaged by the scanner. Thus, the inhaled gas is directly imaged as opposed to the tissue itself. This technique has been used to assess lung ventilation across many diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, idiopathic pulmonary fibrosis, coronavirus disease 2019 (COVID-19), and many others3. In December 2022, HP 129Xe MRI was approved by the United States FDA as an MRI ventilation contrast agent to be used in the United States of America (USA) in adults and pediatric patients aged 12 years and older7. Physicians can now use 129Xe MRI to better care for patients with improved/personalized treatment plans.
Historically, clinical MRI focuses exclusively on imaging hydrogen nuclei (protons) which are abundant in nearly all human viscera. The MRI scanners, sequences, and quality control are generally maintained by the scanner manufacturer as part of the site license and warranty. However, 129Xe requires a multinuclear capable MR scanner and has required a dedicated research team to operationalize the hyperpolarizer, custom-built radiofrequency (RF) coils, dedicated pulse sequences, and offline reconstruction/analysis software. Each of these components can be supplied by third-party vendors or developed in-house. Thus, the burden of quality control generally rests on the 129Xe research team as opposed to the scanner manufacturer or individual third party. Consistent acquisition of high-quality 129Xe data is therefore uniquely challenging as each component of the 129Xe MRI process introduces the potential for error, which must be closely monitored by the 129Xe team. Not only can these situations be extremely frustrating as researchers have to troubleshoot and investigate possible causes for any challenges that may have arisen, but they can be very costly as this slows down patient imaging and subject recruitment. Some costs associated with troubleshooting involve MRI time costs, the hyperpolarization of 129Xe, which involves the consumption of different gases, and the use of materials. Additionally, with the recent FDA approval and growth in 129Xe imaging, providing a standardized protocol for quality control is necessary to avoid common issues and setbacks in 129Xe operation8,9.
Here, we present some of the more commonly encountered issues in 129Xe MRI, including RF coil failures, the emergence of various noise profiles that lead to low signal-to-noise ratio (SNR), and poor quality images10. We aim to provide some concise quality control (QC) guidelines and protocols to ensure the acquisition of high-quality image data and troubleshoot some of the more common issues that can arise in 129Xe MRI. The insights provided here are also relevant for hyperpolarized helium-3 troubleshooting.
Protocol
The protocol outlined below adheres to the guidelines and standards established by the University of Missouri Human Research Ethics Committee, ensuring the ethical conduct of the study and the protection of participants' rights, safety, and well-being.
NOTE: To ensure the reliability and accuracy of hyperpolarized xenon MRI studies, it is crucial to perform rigorous characterization of acquired images, follow a comprehensive protocol, and employ effective troubleshooting strategies. The imaging session involves several steps: gas hyperpolarization, 129Xe coil/scanner communication, 129Xe spectroscopy, acquiring data, data reconstruction, and image analysis.The protocol begins by discussing these steps in detail and highlights the necessary precautions and troubleshooting strategies to optimize the imaging process. By following these procedures and incorporating expert troubleshooting strategies, researchers can optimize the imaging process and overcome challenges that may arise during hyperpolarized xenon MRI studies. Then we will address common troubleshooting practices that may arise in several cases of sub-optimal data.
1. Key steps for a comprehensive HPG MRI study
Here we presented a brief overview of processes involved in a typical hyperpolarized 129Xe imaging session. Detailed protocol recommendations from the 129Xe Clinical Trials Consortium are given in Niedbalski et al.11.
- 129Xe Hyperpolarization
- Ensure the 129Xe hyperpolarizer is set up and operational according to the manufacturer's guidelines or lab-specific protocols for custom-made polarizers.
- Conduct T1 relaxation measurements using the nuclear magnetic resonance (NMR) technique on a representative sample of the HP 129Xe gas at the HP measurement station. In a stable field of 30 mT, xenon in a 1 L gas dose bag should have a T1 of > 45 min.
NOTE: After completing the polarization measurement, the HP 129Xe dose bag should be kept within the magnetic field of the HP measurement station to maintain its polarization until it is ready for transportation to the MR scanner. The polarization will decay according to12,
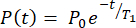 (1.1)
(1.1)
where P(t) is polarization at time t, P0 is initial polarization, and T1 is the magnetization decay rate (not considering polarization losses due to excitation).
- Measuring polarization loss due to gas transport
- Ensure a direct and efficient route from the xenon collection point to the magnet room where the imaging will take place.
- Minimize any delays during HP xenon transportation to maintain polarization, as polarization will decay rapidly once the dose is outside the T1-preserving magnetic field.If the polarization decreases by 20% or more during transportation, use a magnetic-shielded suitcase.
- Avoid extraneous RF signals along the transportation route (e.g., card reader, laser, stainless steel board etc.), as they can contribute to polarization loss.
- Measure the initial dose equivalent (DE) of the HP 129Xe gas before transportation. DE is given by 11,
 (1.2)
(1.2)
where f129 is the isotropic fraction of 129Xe, P129 is 129Xe nuclear spin polarization, and VXe is the total volume of xenon gas. - Transport the gas from the measurement station into the magnet bore, then back along the same route to the polarimetry station. Measure DE again after the round trip to quantify the anticipated signal loss during gas transport. If no additional RF signals interfere along the transportation route, the estimated polarization will closely follow the T1 decay curve outlined in equation 1.1.
- Multinuclear (129Xe MRI) coil
- Place the 129Xe coil correctly in the magnet to ensure proper orientation. If a quadrature coil is used, avoid anti-quadrature excitation, as it can cause a significant signal drop-off in the center of the imaging volume.
NOTE: Xenon coil should accommodate a wide range of chest sizes to accommodate variations in coil tuning/loading between subjects and during different respiration phases, leading to variable delivered flip angles across scans. - Establish a secure physical connection between the coil plug and the MR system through the designated socket and configure the coil software to specify the permissible nuclei (129Xe in our case).
- Divide the well-characterized proton resonance frequency on the MR scanner by 3.61529 to obtain the xenon frequency11.
- Characterize the coil parameters (maximum transmit amplitude, transmitter reference amplitude, specific absorption rate- SAR).
- Place the 129Xe coil correctly in the magnet to ensure proper orientation. If a quadrature coil is used, avoid anti-quadrature excitation, as it can cause a significant signal drop-off in the center of the imaging volume.
- Measuring 129Xe spectroscopy
- Create a thermally polarized 129-xenon phantom.
- Connect a glass pressure vessel to a xenon gas-filled bag, ensuring an appropriate bag size and xenon volume to align with the vessel's capacity.
- Submerge the pressure vessel in a small amount of liquid nitrogen (LN2) to allow xenon diffusion and freezing (see Figure 1).
- Seal the vessel after the xenon has formed frozen snow inside, then allow it to thaw, pressurizing the vessel. Calculate the pressure in the vessel: P = (Vvessel + Vbag)/Vvessel where Vvessel is the volume of the vessel and Vbag is the volume of the xenon in the bag.
NOTE: Unlike hyperpolarized gas (HPG) bags, the thermally polarized 129Xe vessel does not need to be purged of oxygen or vacuum evacuated as the additional oxygen will reduce the xenon T1- a favorable effect in the thermally-polarized phantom. Also, it is important to ensure that the gas pressure in the vessel will not exceed the manufacturer's stated pressure limit. With a phantom of 129Xe gas, the xenon frequency can be measured on the MRI console. Commercial xenon phantoms for quality assurance are also available13.
- Detect the peak frequency with a thermally polarized xenon phantom.
- Put the xenon phantom inside the 129Xe coil and place it similar to that of a loaded patient, as differences in coil geometry can substantially alter the delivered B1 to the phantom (Figure 2).
NOTE: It is recommended that a suitable water phantom be loaded as well to properly load the coil. - Perform a scan with proton frequency, as some scanners may disallow multinuclear scans without an initial proton frequency localizer.
- Use a broadband transmit pulse (if available), high-bandwidth, and high-resolution readout experiment to accurately detect the xenon frequency peak. A broadband pulse will excite a high range of frequencies, ensuring the xenon NMR can be detected.
- Once a well-defined peak is detected, record the frequency to full precision and repeat the experiment at the new frequency with low bandwidth (~1000 Hz) to maximize signal-to-noise ratio(SNR) and peak frequency precision (Figure 3).
- Once a satisfactory, high-signal peak is detected, save the protocol for future QC tests.
NOTE: The precise geometric placement of the coil in the scanner provides a baseline spectroscopy scan, which can be replicated in the future to identify emergent issues if SNR is seen to worsen. The phantom itself can be directly imaged, though it may require multiple acquisitions to build enough signal for image reconstruction and may not provide a fair estimate of achievable SNR as higher flip angles are generally required. A prepared bag of hyperpolarized xenon is the best option for testing the desired imaging protocol with in vivo imaging parameters.
- Put the xenon phantom inside the 129Xe coil and place it similar to that of a loaded patient, as differences in coil geometry can substantially alter the delivered B1 to the phantom (Figure 2).
- Create a thermally polarized 129-xenon phantom.
- HP 129Xe imaging with a test bag
- Use a small amount of HP 129Xe (>300 mL) for imaging, which is well-concentrated and free of oxygen.
- Measure the 129Xe DE accurately immediately prior to imaging.
- Set the test imaging protocol to reflect desired in vivo parameters as closely as possible11.
- Acquire and save the image of the xenon bag as a baseline measure of scanner performance.
- Measure and record the SNR of the acquired images alongside all scan parameters and xenon DE. The acceptable SNR for a 2D GRE scan may vary by site, but it should typically be around 30 or higher, with a minimum threshold of 15 for subsequent image analysis11.
- For measuring flip angle (FA), α, perform a full-volume spoiled gradient-echo scan in which the FOV is imaged twice in succession (with FA ≈ 8-10°), using identical sequence parameters and with no gap between the end of the first image and the start of the second. Measure the SNR at the DC offset of the two images, S0 and S1, count the number of phase encode steps, n, and calculate the flip angle map as follows 14:
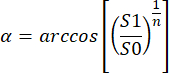 (1.3)
(1.3)
NOTES: Common parameters for in vivo HP 129Xe MRI, as well as a more complicated but highly accurate flip-angle calibration method (multi-shot pulse/acquire experiment), are given in Niedbalski et al.11.
- In vivo HP 129Xe imaging
- Provide proper coaching to the subject regarding breath-holding techniques and allow the subject to practice the inhalation procedure using a bag of air before introducing the HP 129Xe bag.
- Instruct the subject to perform a series of breaths in and out with room air, followed by a deep inhalation of HP 129Xe gas, breath-holding, and initiation of the scan (a commonly used method). Closely monitor the subject's chest movement to ensure that breathing remains synchronized with provided instructions.
NOTE: Various coaching methods are currently employed for breath-hold procedures, and a future consortium paper will likely establish a consensus statement on this. - Use nose clips to prevent nasal inhalation of the gas during breath-holding.
- After breath-hold imaging, coach subjects to take deep breaths to remove xenon from the lungs and resolve any temporary side effects11.
- For those pursuing dissolved-phase xenon imaging, be aware that subject inhalation volume likely impacts the acquired dissolved-phase data substantially15.
- Data reconstruction and analysis
- Export 'raw' data from the scanner, typically in the form of a list of complex data in order of readout acquisition.
- For rectilinearly acquired k-space trajectories, each complex data point corresponds to an integer frequency in two-dimensional (2D) or three-dimensional (3D) k-space. Reconstruct the image using a simple, fast Fourier transform (FFT) for rectilinear trajectories.
- For non-rectilinear trajectories (e.g., radial or spiral data), perform data 'gridding' to interpolate or convolve complex data into integer bins before the subsequent FFT. Examine the data before performing gridding, if necessary, to ensure accuracy and avoid potential artifacts.
NOTE: FFT of raw k-space data may yield images similar but not identical to scanner-reconstructed DICOM images since the scanner further corrects reconstructed images based on known non-linearities in gradient behavior. These effects are generally small, but they can be more pronounced at the edges of the scanner imaging volume, especially when large organs like lungs are imaged. It is recommended to use the scanner-reconstructed image (if available) for post-processing.
2. Troubleshooting steps
NOTE: While the protocol outlined some quality control (QC) procedures in hyperpolarized 129Xe MRI, troubleshooting may be necessary due to emergent issues, anomalies, and challenges. Any errors or missteps in the process can have a ripple effect, impacting subsequent steps and leading to issues such as missing or low-quality images with low signal intensity, high noise levels, or complete signal loss. To address these challenges, strategic approaches should be employed to identify and investigate the problems in detail.
- HP 129Xe dose bag preparation for QC
- Carefully brew a precise amount of xenon gas for the control xenon bag, taking note of any nitrogen mixed with it.
- Image the xenon bag in the MRI scanner and conduct accurate polarization measurements before and after the imaging session for reliable comparisons.
- Use the same imaging sequence for all QC scans to facilitate reliable comparisons.
- Note xenon DE values before and after conducting all QC scans to enable future comparisons.
- Systemic noise characterization
- Create a control noise profile for QC purposes. Use a specific customized 2D GRE sequence that includes a high field of view (FOV; 400-500 mm) to capture the maximum signal from the area, a high Bandwidth per pixel (the maximum available or at least >50 kHz) to identify nearby noise resonances, and the lowest possible repetition time (TR) and echo time (TE)11,13. Acquire the QC for noise profile using a xenon vest or a loop coil.
- Obtain an image with no sample (HP 129Xe) in the coil. This image will characterize the noise profile.
- Examine the acquired noise data, particularly the k-space, for non-Gaussian elements such as spikes, patterns, or discretized/binned values.
- Create a QQ plot by plotting the acquired real/imaginary data against a synthesized Gaussian dataset (with appropriate random number generation function) with identical mean, standard deviation, and vector length, both ordered from smallest to largest. Deviations from the line y = x in the QQ plot indicate the presence of non-Gaussian components within the acquired data, requiring further investigation. (Figure 4).
NOTE: A quantile-quantile plot (QQ plot) can provide insights into whether two datasets exhibit similar distributions. Comparing the data with a normally distributed dataset enables assessment of whether the distribution is Gaussian or not. The protocol assumes that the real and imaginary part of k-space approximates a Gaussian distribution in the absence of a sample. - Identify the noise distribution pattern and potential outliers with a suitable plot of choice (use Chauvenet's criterion if necessary16).
- Categorize noise into regular and irregular types based on its characteristics (see steps 2.3 and 2.4).
NOTE: Regular noise involves regularly appearing patterns in the readout or k-space data. Irregular noise appears relatively random and often has high intensity with no discernible timing pattern but does not demonstrate a Gaussian profile like unavoidable thermal noise.
- Regular noise detection
- To rule out the scanner as a noise source,acquire images using the standard site protocol with various pulse sequence parameters disabled and electronic components powered off. For example, if a particular gradient coil is emitting noise, the gradients should be powered down prior to running the scan to examine whether the noise resolves.
NOTE: Powering down the gradient generally requires elevated access to the scanner console and may require a service engineer to be present. Ultimately, a sequence in which the multinuclear spectrometer is active, but no gradients are powered, and no RF delivered should be sufficient to determine whether a noise issue originates within these components. - Eliminate noise sources from the room and subsequently identify potential origins of regular noise.
NOTE: Noise sources may include electronic components like contrast injectors, code buttons, sensors, vital-sign monitors, scanner components (e.g., positioning laser, bed mechanoelectronics, fans, lights), or waveguides between console/magnet walls. - Use a simple surface loop coil tuned to the 129Xe frequency to 'sniff' around the magnet room for noise sources. Physically place the xenon coil element near potential problematic devices and run a test sequence (see step 2.2.1) to detect amplified noise.
- Examine k-space and image data to pinpoint the exact source of coherence noise.
- If a specific source is identified, attempt to disable it or cover it with aluminum foil/flashing or a copper mesh to reduce noise.
- Rerun the scan after disabling or covering noise sources to see if the noise resolves.Continue this process until all noise sources are eliminated, leaving only low root mean square (RMS) Gaussian noise.
- To rule out the scanner as a noise source,acquire images using the standard site protocol with various pulse sequence parameters disabled and electronic components powered off. For example, if a particular gradient coil is emitting noise, the gradients should be powered down prior to running the scan to examine whether the noise resolves.
- Irregular noise detection
- Identify irregular noise as high signal 'spikes' in individual k-space pixels with abnormally high or low signals in the real or imaginary channels.
NOTE: K-space spikes often result in images with stripe or 'corduroy' patterns (Figure 5). The presence of high values or spikes in the k-space data can often lead to the occurrence of a striped pattern in the image space. This phenomenon is frequently associated with gradient-related issues. - Eliminate potential issues with X, Y, or Z gradients by identifying the direction responsible for the striped pattern (Figure 5). Perform imaging in different phase encoding orientations, including anterior to posterior, head to foot, and left to right.
- Systematically examine the resulting images in each orientation to identify which specific gradient direction is contributing to the striped pattern. If needed, contact the site's clinical engineer to selectively enable and disable individual gradients, allowing for the identification of the source of any noise spikes.
- Identify irregular noise as high signal 'spikes' in individual k-space pixels with abnormally high or low signals in the real or imaginary channels.
- No signal
NOTE: When encountering a situation where no signal is observed after the acquisition in HPG MRI studies, a systematic troubleshooting approach can be undertaken. Here are some recommendations to address this issue,- Verify the xenon coil and connection.
- Ensure that the xenon coil is selected in the MRI scanner and properly connected.
- Movement of the patient during the scan may cause disconnection of the coil, so carefully inspect the coil connection.
- Check if the door of the MRI scanner is securely closed, as an open door can allow outside RF into the magnet room.
- Perform spectroscopy on the xenon phantom (see section 1.4.2) and verify the Xenon peak height and noise floor from the spectroscopy. Use a 90° flip angle to ensure the presence of a xenon peak. Calculate the maximum signal associated with 90° excitation and compare the voltage/power with the QC scan results.
- Evaluate the xenon coil.
- Prepare a small bag of xenon and measure the polarization at the measurement station.
- Image the bag with a simple 2D GRE scan on an HP 129Xe bag with the following parameters: higher flip angle of 90° (adjust the pulse duration if necessary as pulse duration dictates the transmit bandwidth [BW]), use reference voltage based on the previous QC of a phantom, a high FOV and low BW, while keeping the base resolution low.
- Measure the polarization again at the measurement station. If the polarization does not decrease significantly, it suggests a potential issue with the xenon coil transmitter or amplifier.
NOTE: The polarization level experiences a gradual decline due to T1 decay throughout this process, irrespective of the success of excitation pulses from the xenon vest coil. Therefore, a high 90° FA is suggested to observe a sufficient polarization decay caused by the excitation pulse to rule out xenon coil transmitter functionality issue. If the polarization decreases significantly, but no signal is detected in the image, a xenon coil receiver issue is indicated.
- Comprehensive analysis
- Analyze both the k-space and image space data to examine any abnormalities or inconsistencies.
- Compare the acquired data with previous scans or reference data to identify potential differences or deviations.
- Verify the xenon coil and connection.
- Discretization of the data
- Check for data discretization (Figure 6).
NOTE: When coil voltages are recorded by the scanner spectrometer, they are amplified to appropriate levels to ensure the full dynamic range of the spectrometer is employed and the highest fidelity is achieved. The signal is discretized temporally according to the readout bandwidth, which is inversely proportional to datapoint dwell time, and the recorded analog voltage values are digitized into discrete signal 'bins' determined by the spectrometer bit depth. Proper amplification of the incoming signal to span the full bit depth requires the user to have provided correct coil voltage/amplification/scaling values. On some scanners, an imaging scan will be disallowed until preparation pulses are performed at the target frequency - a process that must be avoided for hyperpolarized studies as the additional rf will reduce polarization and increase breath hold time. If the spectrometer is improperly calibrated or fails to adequately amplify the signal, the recorded data may be coarsely discretized - only a small percentage of the amplitude bins are populated with digitized data points. Data discretization can also affect the information content by introducing quantization errors and loss of fine detail. Data discretization can also introduce artifacts, compromise SNR, and limit the ability to accurately analyze physiological changes. Importantly, coarse discretization of k-space data may not prohibit the production of a seemingly satisfactory image (Figure 6). - Optimize the acquisition parameters and employ appropriate reconstruction algorithms to reduce data discretization.
- Improve hardware and utilize techniques like higher sampling rates, advanced interpolation methods, and noise reduction strategies to mitigate the negative effects of data discretization.
- Check for data discretization (Figure 6).
Results
Figure 4 depicts the results of the noise characterization analysis performed on the noise scan. The plot demonstrates the impact of both regular and irregular noise on the k-space, where the deviation from the ideal y=x reference line is observed. Regular noise leads to a continuous pattern in the k-space, while irregular noise results in high-value outliers in the QQ plot.
Moving on to Figure 5, a series of lung images acquired...
Discussion
The ability to troubleshoot 129Xe MRI issues is a necessary skill and may help mitigate problems in real time. Until a hyperpolarized gas infrastructure can be purchased from a single party and garner support from scanner manufacturers, these quality control tasks are the sole responsibility of the individual laboratories. The goal of this manuscript is to provide the reader with helpful practices and suggestions for the inevitable event of poor data acquisition. While we attempt to address as many potential i...
Disclosures
Robert Thomen has provided consulting to Polarean, LLC.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| Polarization measurement station | Polerean | 42881 | https://polarean.com/ |
| Pressure vessele with plunger valve | Ace glass | 8648-85 | https://www.aceglass.com/html/3dissues/Pressure_Vessels/offline/download.pdf |
| Tedlar bag | Jensen inert | GST381S-0707TJO | http://www.jenseninert.com/ |
| Xenon Hyperpolarizer 9820 | Polerean | 49820 | https://polarean.com/ |
| Xenon loop coil | Clinical MR Solutions | Custom device | https://www.sbir.gov/sbc/clinical-mr-solutions-llc |
| Xenon vest coil | Clinical MR Solutions | Custom device | https://www.sbir.gov/sbc/clinical-mr-solutions-llc |
References
- Pellegrino, R., et al. Interpretative strategies for lung function tests. European Respiratory Journal. 26 (5), 948-968 (2005).
- Ebner, L., et al. Hyperpolarized 129Xenon MRI to quantify regional ventilation differences in mild to moderate asthma: A prospective comparison between semi-automated ventilation defect percentage calculation and pulmonary function tests. Investigative Radiology. 52 (2), 120-127 (2017).
- Abuelhia, E., Alghamdi, A. Evaluation of arising exposure of ionizing radiation from computed tomography and the associated health concerns. Journal of Radiation Research and Applied Sciences. 13 (1), 295-300 (2020).
- Kern, A. L., Vogel-Claussen, J. Hyperpolarized gas MRI in pulmonology. The British Journal of Radiology. 91 (1084), 20170647 (2018).
- Möller, H. E., et al. MRI of the lungs using hyperpolarized noble gases. Magnetic Resonance in Medicine. 47 (6), 1029-1051 (2002).
- Salerno, M., Altes, T. A., Mugler, J. P., Nakatsu, M., Hatabu, H., de Lange, E. E. Hyperpolarized noble gas MR imaging of the lung: Potential clinical applications. European Journal of Radiology. 40 (1), 33-44 (2001).
- . New Drug Therapy Approvals at 2022 Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2022 (2023)
- Nikolaou, P., et al. Near-unity nuclear polarization with an open-source 129Xe hyperpolarizer for NMR and MRI. Proceedings of the National Academy of Sciences. 110 (35), 14150-14155 (2013).
- Birchall, J. R., et al. XeUS: A second-generation automated open-source batch-mode clinical-scale hyperpolarizer. Journal of Magnetic Resonance. 319, 106813 (2020).
- He, M., Zha, W., Tan, F., Rankine, L., Fain, S., Driehuys, B. A comparison of two hyperpolarized 129Xe MRI ventilation quantification pipelines: The effect of signal to noise ratio. Academic Radiology. 26 (7), 949-959 (2019).
- Niedbalski, P. J., et al. Protocols for multi-site trials using hyperpolarized 129 Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the 129 Xe MRI clinical trials consortium. Magnetic Resonance in Medicine. 86 (6), 2966-2986 (2021).
- Möller, H. E., et al. MRI of the lungs using hyperpolarized noble gases. Magnetic Resonance in Medicine. 47 (6), 1029-1051 (2002).
- Bier, E. A., et al. A thermally polarized 129Xe phantom for quality assurance in multi-center hyperpolarized gas MRI studies. Magnetic Resonance in Medicine. 82 (5), 1961-1968 (2019).
- Wild, J. M., et al. Comparison between 2D and 3D gradient-echo sequences for MRI of human lung ventilation with hyperpolarized 3He. Magnetic Resonance in Medicine. 52 (3), 673-678 (2004).
- Garrison, W. J., et al. Lung volume dependence and repeatability of hyperpolarized 129Xe MRI gas uptake metrics in healthy volunteers and participants with COPD. Radiology: Cardiothoracic Imaging. 5 (3), e220096 (2023).
- Ni, W., Qi, J., Liu, L., Li, S. A pulse signal preprocessing method based on the Chauvenet criterion. Computational and Mathematical Methods in Medicine. 2019, 2067196 (2019).
- . Available from: https://www.129xectc.org (2023)
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved