A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Measurement of Poly A Tail Length from Drosophila Larva Brain and Cell Line
In This Article
Summary
The protocol describes an efficient and reliable method for quantifying the poly(A) length of the gene of interest from the Drosophila nervous system, which can be easily adapted to tissues or cell types from other species.
Abstract
Polyadenylation is a crucial posttranscriptional modification that adds poly(A) tails to the 3' end of mRNA molecules. The length of the poly(A) tail is tightly regulated by cellular processes. Dysregulation of mRNA polyadenylation has been associated with abnormal gene expression and various diseases, including cancer, neurological disorders, and developmental abnormalities. Therefore, comprehending the dynamics of polyadenylation is vital for unraveling the complexities of mRNA processing and posttranscriptional gene regulation.
This paper presents a method for measuring poly(A) tail lengths in RNA samples isolated from Drosophila larval brains and Drosophila Schneider S2 cells. We employed the guanosine/inosine (G/I) tailing approach, which involves the enzymatic addition of G/I residues at the 3' end of mRNA using yeast poly(A) polymerase. This modification protects the RNA's 3' end from enzymatic degradation. The protected full-length poly(A) tails are then reverse-transcribed using a universal antisense primer. Subsequently, PCR amplification is performed using a gene-specific oligo that targets the gene of interest, along with a universal sequence oligo used for reverse transcription.
This generates PCR products encompassing the poly(A) tails of the gene of interest. Since polyadenylation is not a uniform modification and results in tails of varying lengths, the PCR products display a range of sizes, leading to a smear pattern on agarose gel. Finally, the PCR products are subjected to high-resolution capillary gel electrophoresis, followed by quantification using the sizes of the poly(A) PCR products and the gene-specific PCR product. This technique offers a straightforward and reliable tool for analyzing poly(A) tail lengths, enabling us to gain deeper insights into the intricate mechanisms governing mRNA regulation.
Introduction
Most eukaryotic mRNAs are posttranscriptionally polyadenylated at their 3′ terminus in the nucleus by the addition of non-templated adenosines by canonical poly(A) polymerases. An intact poly(A) tail is pivotal throughout the lifecycle of mRNA, as it is essential for mRNA nuclear export1, facilitates interaction with poly(A)-binding proteins to enhance translational efficiency2, and imparts resistance against degradation3. In certain cases, the poly(A) tail can also undergo extension in the cytoplasm, facilitated by noncanonical poly(A) polymerases4. In the cytoplasm, poly (A) tail length dynamically changes and influences the life span of the mRNA molecule. Numerous polymerases and deadenylases are known for modulating tail length5,6,7. For example, the shortening of poly(A) tails correlates with translational repression, whereas the lengthening of poly(A) tails enhances translation8,9.
Accumulating genomic studies have demonstrated the fundamental significance of the poly(A) tail length across various facets of eukaryotic biology. This includes roles in germ-cell development, early embryonic development, neuronal synaptic plasticity for learning and memory, and the inflammatory response10. There have been numerous methods and assays developed for measuring poly(A) tail lengths. For example, the RNase H/oligo(dT) assay takes advantage of RNase H in the presence or absence of oligo(dT) to study poly(A) tail length11,12. Other methods to study poly(A) tail include the PCR amplification of 3' ends such as rapid amplification of cDNA ends poly(A) test (RACE-PAT)12,13 and the ligase-mediated poly(A) test (LM-PAT)14. Further modifications of the PAT assay include ePAT15 and sPAT16. Enzymatic G-tailing17,18 or G/I-tailing of the 3' end are other variations of the PAT assay. Further modification of these techniques includes the use of fluorescently labeled primers along with capillary gel electrophoresis for high-resolution analysis, referred to as the high-resolution poly(A) test (Hire-PAT)19. These PCR-driven assays allow fast and high-sensitivity poly(A) length quantitation.
With the development of next-generation sequencing, a high-throughput sequencing method, such as PAL-seq20 and TAIL-seq21, allows polyadenylation analyses at a transcriptome-wide scale. However, these methods provide only short sequencing reads of 36-51 nucleotides. Therefore, FLAM-Seq22 was developed for global tail length profiling of full-length mRNA and provides long reads. Nanopore technology23 provides PCR-independent, direct RNA, or direct cDNA sequencing for poly(A) tail length estimations. However, these high-throughput methods are not without limitations. They require large amounts of starting materials, are expensive, and time-consuming. Moreover, analyzing rare transcripts can be extremely challenging with high-throughput methods, and low-throughput PCR-based methods still provide an advantage when a small number of transcripts need to be analyzed, for pilot experiments, and validation of other methods.
We have recently demonstrated that Dscam1 mRNAs contain short poly(A) tails in Drosophila, which necessitates a non-canonical binding of the cytoplasmic poly(A)-binding protein on Dscam1 3'UTR using the G/I tailing method24. Here we provide a streamlined procedure for tissue preparation and quantifying poly(A) length of mRNAs from the Drosophila nervous system and Drosophila S2 cells.
Protocol
1. Rearing and selecting Drosophila larvae
- Maintain/culture the fly strain (w1118, wildtype) on standard fly food medium at 25 ˚C in a humidified incubator.
- Select 10 wandering 3rd instar larvae 72 h after egg laying.
- Place the larvae in a 35 mm empty Petri dish and gently wash them by transferring the larvae to the new dish containing tap water using forceps. Do this 2x to remove any remaining food.
2. Brain isolation from Drosophila larvae (Figure 1)
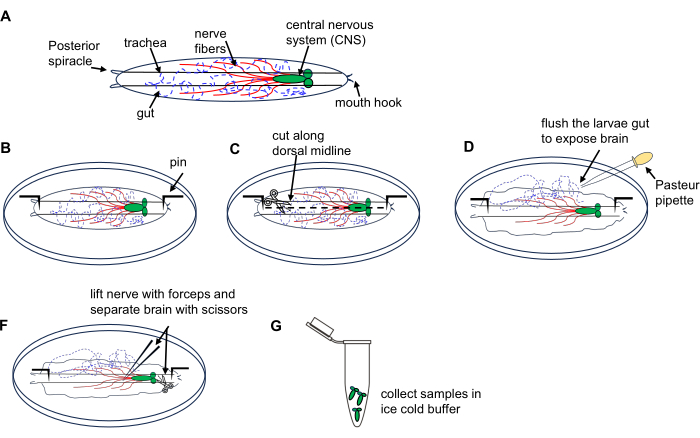
Figure 1: Dissection of Drosophila larval brain from 3rd instar wandering stage. (A) Schematic drawings of Drosophila larva. (B-G) Larva dissection. Please click here to view a larger version of this figure.
- Place 10 larvae on a dissection dish containing ice-cold PBS.
- Place the larva dorsal side up (identified by tracheal tubes running along its length) and pin each end to the bottom of the dish followed by making a small incision on the body wall at the posterior end.
- Cut the body wall along the dorsal midline towards the anterior end using microdissection scissors.
- Briefly flush the interior of the larva using a Pasteur pipette 3x with PBS in the dish to expose the brain.
- Locate and lift the brain using the forceps and carefully isolate it using microdissection scissors.
- Transfer the dissected brains to a 1.5 mL microcentrifuge tube filled with ice-cold PBS on ice; collect all larva brains. Proceed with RNA extraction using an RNA microprep kit as described in section 4.
NOTE: Do a dissection of 10 larvae within 15 min to prevent tissue damage and RNA degradation.
3. Drosophila S2 Schneider cells
- Grow Drosophila S2 cells in Drosophila Schneider's medium supplemented with 10% fetal bovine serum (FBS) at 25 °C in a humidified incubator to a density of 8 × 106 to 10 × 106 cells/mL with minimum 90% viability.
- In a 50 mL sterile conical tube, dilute cells to 2.5 × 106 cells/mL with Schneider's Drosophila medium supplemented with 10% FBS that has been prewarmed to 25 °C.
- Transfer 8 mL of the cell suspension (20 × 106 cells) into a 100 mm culture plate and add 4 mL of medium to make it to 12 mL (day 1).
- Incubate the cultured cells at 25 °C in a humidified incubator.
NOTE: Cells loosely adhere to the plate after 12-16 h (day 2). - Transfect the cells with appropriate DNA plasmids24.
- Incubate for 48 h in a humidified incubator.
- After incubation, collect cells by adding 5 mL of ice-cold PBS by gentle pipetting (day 4).
- Transfer the cells to a 15 mL tube.
- Pellet the cells by centrifuging at 1,000 × g for 5 min at 4 °C.
- Rinse the cells 2x with ice-cold PBS by gentle pipetting and collect the cells by centrifuging them at 1,000 × g for 5 min at 4 °C.
- Perform RNA extraction using an RNA miniprep kit.
NOTE: Perform the following steps inside a sterile laminar flow hood.
4. Total RNA extraction from Drosophila larvae brain and S2 cells
- Larval brain: Remove PBS by brief centrifugation (8 s short spin at 5,000 × g).
- Add 600 µL of RNA lysis buffer and homogenize 10x with a plastic pestle. Visually inspect the tube under a stereo microscope to ensure complete lysis.
- Centrifuge at 1,000 × g for 5 min at 4 °C to remove tissue debris. Transfer the cleared supernatant into a nuclease-free microcentrifuge tube.
- Isolate RNA using an RNA microprep kit according to the manufacturer's instructions.
NOTE: The use of an RNA microprep kit is essential for the larval brain samples because of the small amount of RNA present in the samples. - S2 cells: Remove PBS and isolate RNA according to the manufacturer's instructions.
- Measure RNA yield and quality by spectrophotometry and agarose gel electrophoresis.
- Determine the purity and quantity of extracted RNA by measuring the optical density of the extracted RNA at A260 nm and A280 nm, respectively. Make sure the A260 nm/A280 nm ratio is ≥2.0 and the RNA concentration is >350 ng/µL for downstream applications.
NOTE: A typical RNA yield from 10 Drosophila larva brains is ~500-800 ng/µL or 2.5-4 µg in 5 µL. For S2 cells, the yield is ~2-3 µg/µL (15-30 µg in 15 µL). Isolated RNA can be stored at -80 °C for long-term storage.
- Determine the purity and quantity of extracted RNA by measuring the optical density of the extracted RNA at A260 nm and A280 nm, respectively. Make sure the A260 nm/A280 nm ratio is ≥2.0 and the RNA concentration is >350 ng/µL for downstream applications.
5. Preparation of RNA gel and electrophoresis
- 1.5% Denaturing RNA gel (100 mL)
NOTE: Formaldehyde is toxic through skin contact and inhalation of vapors; handle it in a chemical fume hood.- Dissolve three agarose tablets (1.5 g) in 82 mL of MOPS buffer (Supplemental File 1) until tablets completely break up to form fine particles.
- Heat the agarose slurry in a microwave until the solution is clear and all particles are completely dissolved.
- Cool the solution to ~60 °C.
- Add 18 mL of 37% formaldehyde, then mix by gentle swirling. Pour the solution into the casting tray and let it solidify in a fume hood.
- RNA sample preparation and electrophoresis
- Dilute the RNA sample to 200 ng (in 5 µL) and add 5 µL of 2x RNA loading dye.
- Heat the samples at 70 ˚C for 5 min in a dry bath.
- Load 2 µL of RNA ladder in the first lane and 10 µL of samples in the adjacent lanes.
- Perform electrophoresis in MOPS buffer at 100 V for 60 min for a 5 x 6 cm gel.
NOTE: Adjust the electrophoresis conditions depending on the amplicon sizes. - Visualize the gel on a UV transilluminator.
NOTE: The presence of a single band ~600 nucleotide size indicates intact RNA preparation (see Figure 2A).
6. Poly(A) tail length measurement
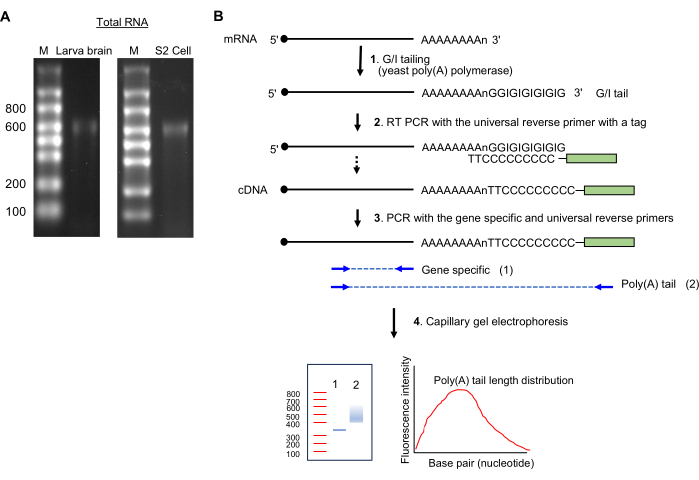
Figure 2: RNA sample preparation and the poly(A)-tail assay. (A) The RNA gel images show total RNA from the Drosophila larva brain (left) and S2 cells (right) on a 1.5% formaldehyde agarose gel. Single-stranded RNA ladder sizes are shown in nucleotides on lane M. Note a major RNA banding at ~600 nt, which is from rRNA. (B) Schematics of poly(A)-tail assay. Abbreviation: G/I = guanosine/inosine. Please click here to view a larger version of this figure.
- GI tailing (Figure 2B)
- Keeping the reagents on ice, prepare the following mixture (20 µL): up to 14 µL of total RNA sample (1 µg), 4 µL of 5x Tail buffer mix, and 2 µL of 10x Tail enzyme mix.
- Incubate at 37 °C for 60 min in a thermocycler.
- Add 1.5 µL of the tail stop solution; keep on ice for 2 min.
NOTE: Proceed to reverse transcription or store the GI-tailed RNA samples at -80 °C until ready to proceed to reverse transcription.
- Reverse transcription and PCR amplification
- Synthesize cDNA by preparing the mix and incubating it under the conditions described in Supplemental File 1.
- Dilute the cDNA samples and perform PCR to amplify DNA as indicated in Supplemental File 1.
7. PCR product analysis by agarose gel electrophoresis
- Analyze a small portion (2-5 µL) of the PCR products from step 6.2.2 on a 2.5% agarose gel by electrophoresis at 100 V for 45 min for quality control.
- Verify the specificity of PCR for gene-specific and tail-specific reactions by sequencing the gel-extracted PCR bands.
8. Capillary electrophoresis
- Perform high-resolution gel electrophoresis on 1 µL of PCR products (0.5-5 ng/µL) from gene-specific and poly(A)-specific PCR using the bioanalyzer with a high-sensitivity DNA kit. Look for well-resolved peaks indicating a successful run.
9. Data analysis: poly(A) tail length measurement (Figure 3)

Figure 3: Poly(A) tail length and peak value measurement. Please click here to view a larger version of this figure.
- Accessing data
- To access the data, open the xad file in the software.
- Select the sample name or the ladder in the tree view panel.
NOTE: This will show the result as electropherograms or gel-like images for the selected samples. The lower marker 35 base pair (bp) and upper marker 10,380 bp are the internal standards used to align the ladder data (50-7,000 bp) with data from the sample wells. - Zoom in and out of the electropherograms and gel-like images to display the details.
- Performing peak and smear analysis
- To obtain the peak sizes, open the electropherogram of a selected sample.
- Right-click on the electropherogram and select manual integration to manually select peaks by dragging the horizontal line.
- Observe the peak values in the Peak table. Identify the peak with the largest Peak Height. This is a peak of poly(A) tail length for an individual sample. In the example shown, it is 346 bp.
- Enable the Show/Hide Setpoints icon on the top menu and wait for a new panel to pop up on the right side.
- Select Advanced, scroll down to find Perform Smear Analysis, and select the check box. This will add the Region table to the electropherogram tab.
- Select an electropherogram from a sample and go to the Region table, which shows the From [bp] and To [bp] menu. To set the starting and ending [bp], right-click on the electropherogram and select Region to add Add Region.
- Right-click on any cells in the Region table and select Modify Regions to make a small new window pop up where custom regions can be set.
NOTE: For example, we used a region of 300 bp to 550 bp for GAPDH. The gene-specific GAPDH PCR yielded a peak at 265 bp. The universal primer (Table 1) extends the length of poly(A) PCR by 35 bp via annealing to G/I-tailed RNAs. Thus, the first adenine nucleotide on GAPDH RNA starts at 300 bp (265 + 35). We arbitrarily limited the maximum poly(A) tail length to 250 (300 + 250 = 550). From the region table, the program returns the average size within the region as 387 bp. - Use equation (1) to calculate the poly(A) tail length on the mRNA of interest:
Poly(A) tail length = (A - B - 35) (1)
Where A is the average bp of poly(A)-specific PCR product from electropherogram (i.e., 387 bp for GAPDH), B is the peak bp of gene-specific PCR product from electropherogram (i.e., 265 bp for GAPDH), and "35" is the length of universal reverse primer tag.
NOTE: From the calculation above, the average poly(A) tail length of GAPDH is 387 - 265 - 35= 87 bp.
10. Visualizing poly(A) tail length distribution
- Export data as a csv file under File | Export | Sample Data to retrieve sample data.
NOTE: The exported csv file displays run time rather than bp on the X-axis. - Go to an electropherogram of a sample and check on Show Sizes on the Electropherogram tab to automatically convert the region table on Region Table from bp to run time. In the example, 70.39 s to 86.28 s correspond to 300 bp to 550 bp.
- Open the csv file and select the values from 70.39 s to 86.28 s of run time to generate a graph. To visualize bp sizes to the X-axis on the graph, export the electropherogram with bp sizes as an image file and overlay it on the graph generated in the spreadsheet. This will appropriately match the bp sizes on poly(A) tail distribution.
Results
Here, we analyzed the poly(A) tail length of Dscam1 and GAPDH from Drosophila larval brains (Figure 4). Isolated RNAs were visualized on an agarose gel for quality control. A single RNA band at around 600 nucleotide size indicates intact RNA preparation (Figure 2A). RNAs were subjected to the G/I tailing and high-resolution capillary electrophoresis using an Agilent 2100 bioanalyzer. The gel images were exported using the Agilent 2100 ...
Discussion
In this protocol, we describe the technique to dissect the Drosophila larval brain from wandering 3rd instar stage as well as the sample preparation from Drosophila S2 cells. Due to the labile nature of mRNAs, sample collection requires extra caution. For larval brain dissection, brains should not be damaged during isolation and should not be kept in solution for a prolonged duration. Keeping dissection time to 8-10 min for a round of dissection is essential. It may also be beneficial to supp...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported by the National Institute of Neurological Disorders and Stroke Grant R01NS116463 to J.K., and the Cellular and Molecular Imaging Core facility at the University of Nevada, Reno, which was supported by National Institutes of Health Grant P20GM103650 and used for research reported in this study.
Materials
| Name | Company | Catalog Number | Comments |
| 3-(N-morpholino) propanesulfonic acid (MOPS) | Research Product Internation (RPI) | M92020 | |
| Agilent High Sensitivity DNA Kit | Agilent Technologies | 5067-4626 | |
| Agilent software 2100 expert free download demo | Agilent Technologies | https://www.agilent.com/en/product/automated-electrophoresis/bioanalyzer-systems/bioanalyzer-software/2100-expert-software-228259 | |
| Apex 100 bp-Low DNA Ladder | Genesee Scientific | 19-109 | |
| Bioanalyzer | Agilent 2100 Bioanalyzer G2938C | ||
| Diethyl pyrocarbonate (DEPC) | Research Product Internation (RPI) | D43060 | |
| DNA dye (Gel Loading Dye, Purple (6x) | New England biolabs | B7024S | |
| Drosophila S2 cell line | Drosophila Genomics Resource Center stock #181 | ||
| Drosophila Schneider’s Medium | Thermo Fisher Scientific | 21720024 | |
| Ehidium bromide | Genesee scientific | 20-276 | |
| Fetal bovine serum (FBS) | Sigma-Aldrich | F4135 | |
| Forceps Dumont 5 | Fine Science tools | 11254-20 | |
| Nuclease free water | Thermo Fisher Scientific | AM9932 | |
| PBS 10x | Research Product Internation (RPI) | P32200 | |
| Poly(A) Tail-Length Assay Kit | Thermo Fisher Scientific | 764551KT | |
| RiboRuler Low Range RNA Ladder | Thermo Fisher Scientific | SM1833 | |
| RNA Gel Loading Dye (2x) | Thermo Fisher Scientific | R0641 | |
| RNA microprep kit | Zymoresearch | R1050 | |
| RNA miniprep kit | Zymoresearch | R1055 | |
| Scissors-Vannas Spring Scissors - 2.5 mm Cutting Edge | Fine Science tools | 15000-08 | |
| TopVision Agarose Tablets | Thermo Fisher Scientific | R2802 | |
| Tris-Acetate-EDTA (TAE) | Thermo Fisher Scientific | B49 |
References
- Stewart, M. Polyadenylation and nuclear export of mRNAs. Journal of Biological Chemistry. 294 (9), 2977-2987 (2019).
- Machida, K., et al. Dynamic interaction of poly(A)-binding protein with the ribosome. Scientific Reports. 8 (1), 17435 (2018).
- Eisen, T. J., et al. The dynamics of cytoplasmic mRNA metabolism. Molecular Cell. 77 (4), 786-799 (2020).
- Liudkovska, V., Dziembowski, A. Functions and mechanisms of RNA tailing by metazoan terminal nucleotidyltransferases. Wiley Interdisciplinary Reviews RNA. 12 (2), e1622 (2021).
- Goldstrohm, A. C., Wickens, M. Multifunctional deadenylase complexes diversify mRNA control. Nature Reviews Molecular Cell Biology. 9 (4), 337-344 (2008).
- Schmidt, M. J., Norbury, C. J. Polyadenylation and beyond: emerging roles for noncanonical poly(A) polymerases. Wiley interdisciplinary reviews RNA. 1 (1), 142-151 (2010).
- Laishram, R. S. Poly(A) polymerase (PAP) diversity in gene expression - Star-PAP vs canonical PAP. FEBS Letters. 588 (14), 2185-2197 (2014).
- Salles, F. J., Lieberfarb, M. E., Wreden, C., Gergen, J. P., Strickland, S. Coordinate initiation of Drosophila development by regulated polyadenylation of maternal messenger RNAs. Science. 266 (5193), 1996-1999 (1994).
- Wreden, C., Verrotti, A. C., Schisa, J. A., Lieberfarb, M. E., Strickland, S. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development. 124 (15), 3015-3023 (1997).
- Passmore, L. A., Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nature Reviews Molecular Cell Biology. 23 (2), 93-106 (2021).
- Murray, E. L., Schoenberg, D. R. Assays for determining poly(a) tail length and the polarity of mRNA decay in mammalian cells. Methods in Enzymology. 448, 483-504 (2008).
- Salles, F. J., Strickland, S. Analysis of poly(a) tail lengths by PCR: The PAT assay. Methods in Molecular Biology. 118, 441-448 (1999).
- Salles, F. J., Darrow, A. L., O'Connell, M. L., Strickland, S. Isolation of novel murine maternal mRNAs regulated by cytoplasmic polyadenylation. Genes and Development. 6 (7), 1202-1212 (1992).
- Salles, F. J., Strickland, S. Rapid and sensitive analysis of mRNA polyadenylation states by PCR. Genome Research. 4 (6), 317-321 (1995).
- Janicke, A., Vancuylenberg, J., Boag, P. R., Traven, A., Beilharz, T. H. ePAT: A simple method to tag adenylated RNA to measure poly(a)-tail length and other 3' RACE applications. RNA. 18 (6), 1289-1295 (2012).
- Minasaki, R., Rudel, D., Eckmann, C. R. Increased sensitivity and accuracy of a single-stranded DNA splint-mediated ligation assay (sPAT) reveals poly(a) tail length dynamics of developmentally regulated mRNAs. RNA Biology. 11 (2), 111-123 (2014).
- Martin, G., Keller, W. Tailing and 3'-end labeling of RNA with yeast poly(A) polymerase and various nucleotides. RNA. 4 (2), 226-230 (1998).
- Kusov, Y. Y., Shatirishvili, G., Dzagurov, G., Verena, G. M. A new G-tailing method for the determination of the poly(a) tail length applied to hepatitis a virus RNA. Nucleic Acids Research. 29 (12), 57 (2001).
- Bazzini, A. A., Lee, M. T., Giraldez, A. J. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 336 (6078), 233-237 (2012).
- Subtelny, A. O., Eichhorn, S. W., Chen, G. R., Sive, H., Bartel, D. P. Poly(a)-tail profiling reveals an embryonic switch in translational control. Nature. 508 (1), 66-71 (2014).
- Chang, H., Lim, J., Ha, M., Kim, V. N. TAIL-seq: Genome-wide determination of poly(a) tail length and 3' end modifications. Molecular Cell. 53 (6), 1044-1052 (2014).
- Legnini, I., Alles, J., Karaiskos, N., Ayoub, S., Rajewsky, N. FLAM-seq: Full-length mRNA sequencing reveals principles of poly(A) tail length control. Nature Methods. 16 (9), 879-886 (2019).
- Garalde, D. R., et al. Highly parallel direct RNA sequencing on an array of nanopores. Nature Methods. 15 (3), 201-206 (2018).
- Singh, M., Ye, B., Kim, J. H. Dual leucine zipper kinase regulates Dscam expression through a noncanonical function of the cytoplasmic poly(A)-binding protein. Journal of Neuroscience. 42 (31), 6007-6019 (2022).
- Macharia, R. W., Ombura, F. L., Aroko, E. O. Insects' RNA profiling reveals absence of "hidden break" in 28S ribosomal RNA molecule of onion thrips, Thrips tabaci. Journal of Nucleic Acids. 2015, 965294 (2015).
- Miura, P., Sanfilippo, P., Shenker, S., Lai, E. C. Alternative polyadenylation in the nervous system: to what lengths will 3' UTR extensions take us. Bioessays. 36 (8), 766-777 (2014).
- Sement, F. M., et al. et al Uridylation prevents 3' trimming of oligoadenylated mRNAs. Nucleic Acids Research. 41 (14), 7115-7127 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved