A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Development of a Low-cost Epimysial Electromyography Electrode: A Simplified Workflow for Fabrication and Testing
In This Article
Summary
Our purpose was to provide an updated, easy-to-follow guide on the fabrication and testing of epimysial electromyography electrodes. To that end, we provide instructions for material sourcing and a detailed walkthrough of the fabrication and testing process.
Abstract
Electromyography (EMG) is a valuable diagnostic tool for detecting neuromuscular abnormalities. Implantable epimysial electrodes are commonly used to measure EMG signals in preclinical models. Although classical resources exist describing the principles of epimysial electrode fabrication, there is a sparsity of illustrative information translating electrode theory to practice. To remedy this, we provide an updated, easy-to-follow guide on fabricating and testing a low-cost epimysial electrode.
Electrodes were made by folding and inserting two platinum-iridium foils into a precut silicone base to form the contact surfaces. Next, coated stainless steel wires were welded to each contact surface to form the electrode leads. Lastly, a silicone mixture was used to seal the electrode. Ex vivo testing was conducted to compare our custom-fabricated electrode to an industry standard electrode in a saline bath, where high levels of signal agreement (sine [intraclass correlation - ICC= 0.993], square [ICC = 0.995], triangle [ICC = 0.958]), and temporal-synchrony (sine [r = 0.987], square [r = 0.990], triangle [r= 0.931]) were found across all waveforms. Low levels of electrode impedance were also quantified via electrochemical impedance spectroscopy.
An in vivo performance assessment was also conducted where the vastus lateralis muscle of a rat was surgically instrumented with the custom-fabricated electrode and signaling was acquired during uphill and downhill walking. As expected, peak EMG activity was significantly lower during downhill walking (0.008 ± 0.005 mV) than uphill (0.031 ± 0.180 mV, p = 0.005), supporting the validity of the device. The reliability and biocompatibility of the device were also supported by consistent signaling during level walking at 14 days and 56 days post implantation (0.01 ± 0.007 mV, 0.012 ± 0.007 mV respectively; p > 0.05) and the absence of histological inflammation. Collectively, we provide an updated workflow for the fabrication and testing of low-cost epimysial electrodes.
Introduction
Electromyography (EMG) is a powerful tool for studying the electrical activity of muscle. EMG recordings can be especially useful in preclinical animal models to assess the effectiveness of interventions to treat neuromuscular dysfunction. In these models, implantable biocompatible electrodes are commonly used to assess the neurophysiological interface between motor neurons and muscle fibers. These implantable electrodes can provide localized measurements of muscle excitation and can be diverse in terms of their configuration, shape, and material, with the optimal design ultimately dictated by the location and intended use.
Despite their suitability for assessing muscle excitation in preclinical models, the use of epimysial electrodes can be limited by cost. As a result, many investigators use custom-fabricated epimysial electrodes that are produced in-house. Although resources exist detailing the fundamental considerations of electrode fabrication, testing, and use1,2, there is a need for an updated instructional guide detailing the sourcing, fabrication, and validation of epimysial electrodes using modern methods. Informed by the foundational works of Loeb and Gans3 and others in electrode theory, we present modern instructions on the sourcing and fabrication of low-cost epimysial electrodes and test their performance in a series of ex vivo and in vivo experiments. The aim is to offer a user-friendly guide for others in the scientific community to source, fabricate, and test in-house low-cost epimysial electrodes for animal use, enabling the broader quantification of muscle excitation in preclinical models.
In this protocol, we provide an instructional guide to the sourcing, fabrication, and testing of epimysial electrodes for animal use in the modern electrophysiology laboratory. Electrode parameters chosen for fabrication, such as the shape, dimensions, contact surface area, interelectrode distance, lead length, etc., were selected to suit our experimental needs and were comparable to a commercially available industry standard epimysial electrode (see the Table of Materials). We encourage other groups to modify these parameters to suit their needs in addition to selecting a reliable industry standard electrode that matches their use case.
In an effort to give readers a relatively quick sense of electrode performance, we also provide an example of an ex vivo testing protocol with the option of measuring electrode impedance. Additionally, we give an example assessment of electrode performance in vivo. The ex vivo experiment compared the custom-fabricated electrode to an industry standard in a saline bath to mimic stable physiological conditions. Impedance was also assessed ex vivo via electrochemical impedance spectroscopy (EIS). The in vivo experiment consisted of the surgical implantation of the custom-fabricated electrode into the vastus lateralis (VL) muscle of a 16-week-old female Long Evans rat (HsdBlu: LE, Envigo) to measure the EMG signal during conditions known to elicit a high or low signal (uphill, downhill walking). To assess the reliability of the custom-fabricated electrode, EMG signaling was acquired during level walking following full surgical recovery and prior to sacrifice (14 days and 56 days post implantation, respectively). Hematoxylin-eosin (H&E) staining was conducted on the instrumented muscle to assess the biocompatibility of the custom-fabricated electrode.
Protocol
The in vivo procedure was conducted under the approval of the Institutional Animal Care & Use Committee at the University of Michigan (IACUC approval #PRO00010765) and in accordance with the National Institutes of Health guidelines on the care and use of laboratory animals.
1. Electrode sourcing and fabrication
NOTE: Figure 1 provides a high-level summary of all key fabrication steps with a QR link that provides additional visual instructions.
- Source biocompatible electrode materials directly from the manufacturer in bulk for fabrication. See the Table of Materials, which contains all electrode components and details on sourcing.
NOTE: Ensure that the silicone base, silicone sealant, and platinum-iridium contact foils are biocompatible to avoid an adverse immune response and allow for chronic implantation. To support the economic advantages of in-house fabrication, we present a detailed cost analysis of the custom-fabricated epimysial electrode in comparison to the industry standard (Table of Materials). - Prepare Cutting and Folding Jig (Supplemental File 1, Supplemental File 2). Use selected 3D printing software to design cutting and folding jigs for the batch production of identical epimysial electrodes.
- Design the cutting jig to ensure consistently sized and spaced perforations of the silicone base for equal contact surface area and interelectrode distance across all electrodes.
- Create the folding jig to fold the platinum-iridium contact surfaces and simplify the placement of the foils in the silicone base.
NOTE: Gcode files are provided; modify the dimensions and specifications of the cutting and folding jig to suit experimental needs.
- Perforate the silicone base. Place the silicone base on the cutting jig and tape it down. Perforate the silicone using the guides in the cutting jig using an X-acto knife for placement of the contact foils.
- Insert the foils into the silicone base to form the contact surfaces. Fold the biocompatible, precut platinum-iridium contact foils (1.25 mm x 5 mm) into a U-shape using the folding jig. Next, guide the arms of the folded foils into the perforated slots in the silicone base to construct the contact surfaces of the electrode. In this manner, insert all 12 foils into the silicone base; leave a single column open between electrodes to ensure proper spacing.
NOTE: This configuration will allow for the fabrication of 6 bipolar epimysial electrodes; editing the cutting jig can expand capacity if needed. - Remove the silicone base from the cutting jig. Place a piece of surgical tape over the contact surfaces to hold the foils in place. Next, remove the silicone base from the cutting jig, flip over so the arms of the U-shaped foil are exposed, and fold one arm of the U-shaped foil flush with the silicone base.
- Connect the electrode leads. Prepare a perfluoroalkoxy (PFA)-coated stainless-steel wire by cutting it to a desired length. Next, denude one end of the stainless-steel wire by ~ 1 cm using a commercially available lighter. Position the denuded end of the stainless-steel wire on the inside of the unfolded arm of the foil. Weld the stainless-steel wire to the arm of the foil using a Micro TIG welder (pulse width = 0, weld energy = 25).
- Inspect the lead-foil interface. Test the wire-foil connection by applying tension to the stainless-steel wire. If the connection holds, fold the arm of the foil down flush with the silicone base and remove the tape bordering the silicone sheet.
NOTE: Apply tension that is representative of the tension applied in the specified use case. - Seal the electrode. To achieve a thinner consistency, mix 1 g of biocompatible liquid silicone with 0.75 mL of toluene. Draw up the mixture using an 18 G blunt-tip syringe and apply it to the welded side of each electrode to seal the back of the electrodes.
CAUTION: Toluene is flammable, may be fatal if swallowed or enters the airways and causes skin irritation. Work under the fume hood and keep away from open flames. - Separate the batch into individual electrodes. Allow the silicone-toluene to set for 72 h before handling the electrode. Next, use scissors to cut the silicone base into individual electrodes with a size of 10 x 5 mm.
NOTE: Electrode size will depend on the dimensions of the cutting jig and the specific use case. Autoclave and/or clean electrodes in an ultrasonic bath.
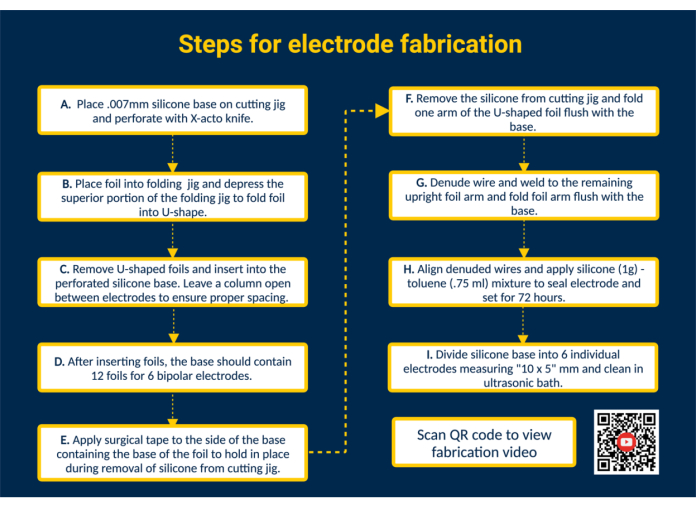
Figure 1. Steps for electrode fabrication. (A) Perforate silicone base. (B) Form U-shaped foils using the folding jig. (C) Insert U-shaped foils into perforated silicone base. (D) Silicone base contains 12 foils to form 6 bipolar electrodes. (E) Apply surgical tape to the base to secure foils during removal from the cutting jig. (F) Remove silicone base from the cutting jig. (G) Denude PFA-coated stainless-steel wire and weld to the upright foil arm using a Micro TIG welder. (H) Align denuded wires, apply silicone-toluene sealant, and let set. (I) Divide the silicone base into individual electrodes and clean in an ultrasonic bath. Please click here to view a larger version of this figure.
2. Ex vivo testing
- Connect electrodes to a recording device. Pin each electrode lead wire to a channel on an electrode interface board (EIB). Repeat this step (using the same EIB) for a selected industry standard electrode for comparison. Connect the EIB to a data acquisition platform via a magnetic tethered cable system.
NOTE: The industry standard electrode can be any electrode deemed reliable and that suits experimental needs. - Ground the EIB. Denude one end of a PFA-coated stainless steel and spot weld to a grounding source (e.g., a stainless-steel screw). Pin the ground lead wire to the same electrode interface board in the designated "ground" position.
- Set up the saline bath. Fill a glass beaker (250 mL) with 180 mL of physiological saline solution (0.9% sterile saline solution)4. Submerge the custom-fabricated and industry standard epimysial electrodes in the saline bath and secure them in a stable position. Next, submerge the grounding source into the saline bath and secure its position. Lastly, submerge and affix two stimulating needle electrodes in the saline bath and connect the stimulating electrodes to the signal generator.
- Use a signal generator to assess signal agreement and temporal synchrony. Use a signal generator to deliver repeated waveforms at a selected voltage and frequency (0.1 V and 5 Hz) into the saline bath via the stimulating electrodes (Figure 2). Deliver various waveforms (sine, square, and triangle) to compare recorded signals between the custom-fabricated epimysial electrode relative to the industry standard.
- Assess the performance informally and visually in real time to assess the degree to which the signals between electrodes vary. Perform intra-class correlations (ICCs) and Pearson correlations to assess signal agreement and temporal synchrony, respectively.
NOTE: For the purposes of our analysis, 8,000 samples were acquired at 4,000 Hz and filtered in real time with a high and low pass Butterworth filter (high pass = 75 Hz; low pass = 2 kHz). - Measure electrode impedance. To measure electrode impedance, collect EIS on the custom-fabricated electrodes (e.g., 10 electrodes) over frequencies 10 Hz-31 kHz5 with a potentiostat using the procedure described below by Richie et al.6. See Sarolic et al. for additional information on measuring electrode impedance on bipolar electrodes7.
- Following the procedure from Richie et al., submerge epimysial electrode 1 mm into 1x phosphate-buffered saline (PBS). Use a silver-silver chloride (Ag|AgCl) reference electrode and a stainless-steel rod as a counter electrode to complete the circuit.
- Suspend the Ag|AgCl reference electrode and the stainless-steel rod in the 1x PBS using a beaker clamp. Connect the reference electrode to the reference of the impedance system being used and connect to the counter electrode input of the impedance system being used.
- Use a potentiostat to run a 1 kHz impedance scan. Set to a 1 kHz scan frequency at 0.01 Vrms in a single sine waveform. During the first 5 seconds of the scan, set the potentiostat to 0 V to stabilize the recorded signal. Use the potentiostat-associated software to record the measurements.
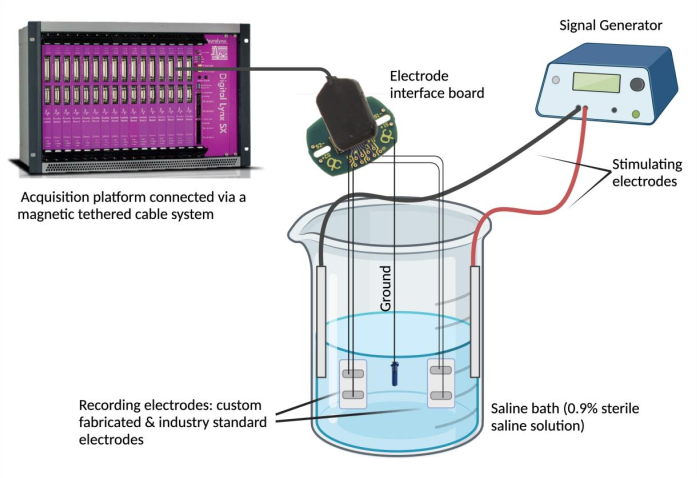
Figure 2: Ex vivo testing: Saline bath containing the custom-fabricated electrode, industry standard electrodes, two stimulating electrodes, and a ground source. Signal agreement and temporal synchrony were assessed by delivering sine, square, and triangle waves into the saline bath from the signal generator and recording the waveforms detected by the respective electrodes using a data acquisition platform. NOTE: Electrochemical impedance spectroscopy is not pictured. Please click here to view a larger version of this figure.
3. In vivo testing
NOTE: The in vivo testing procedure describes our experimental use case. It is recommended that custom-fabricated epimysial electrodes are tested in vivo in a way that matches the user's intended experimental conditions.
- Review surgical information; consult information for surgeons who are learning the process of implanting chronic neural recording electrodes using the following resources8,9.
NOTE: Given the focus of our manuscript is on electrode fabrication and testing, here we only provide a brief overview of surgical techniques used in our laboratory for in vivo electrode testing. - Select a rat to be instrumented (e.g., a female Long Evans rat at 16 weeks of age). Have the rat undergo a 1 week period of laboratory acclimation prior to treadmill acclimation. After laboratory acclimation, gradually expose the rat to a rodent treadmill at increasing speeds up to 16 m/min.
- Prepare the EIB by first attaching a custom-fabricated epimysial electrode to the EIB by pinning each electrode lead to a channel on the EIB. To ground the EIB, denude one end of a PFA-coated stainless-steel wire and spot weld to a selected grounding (e.g., stainless-steel screw). Next, pin the ground lead wire to the same EIB in the designated "ground" position.
NOTE: To protect the electrode during instrumentation, wrap it with a protective covering (e.g., parafilm). - Disinfect all surgical tools as well as the surgical area before beginning. Use sealed, aseptic surgical supplies and autoclave all surgical instruments that are not in sterile packaging. Create a sterile surgical field using a surgical drape.
- Anesthetize the animal using an induction chamber with 3-5% isoflurane and 1 L/min oxygen and maintain via a nose cone with 2% isoflurane and 500 mL oxygen while preparing the rat. After reaching the surgical plane of anesthesia, assessed via toe pinch, apply eye ointment, monitor respiratory rate (70-110 breaths/min), and assess core temperature using a rectal probe.
- Next, shave the surgical site with animal clippers and remove debris from the surgical site. Once the surgical area is shaved, disinfect the surgical site using chlorhexidine, isopropyl alcohol, and povidone-iodine by scrubbing from the center to the outside of the surgical area. Administer analgesics as appropriate and approved and provide heat to maintain core body temperature (37.5-38.5 °C). Following preparation, position the rat in a stereotactic frame and establish the sterile field.
- For proper surgical preparation of the surgeon, thoroughly wash hands using soap and/or a disinfectant (chlorhexidine). Don proper PPE: mask, sterile gloves, and a disposable gown/scrub top. Don new sterile gloves if the aseptic technique is violated for any reason.
- To instrument the right VL, use a scalpel to make a 3-5 cm incision on the anterolateral portion of the right hindlimb and use blunt dissection to identify the VL muscle.
- Make a 1 cm sagittal plane incision along the coronal suture and expose the skull. Using a thin pair of forceps, make a subdermal tunnel from the right hindlimb to the base of the skull. Use the forceps to grab the electrode and route the electrode to the VL.
- Use a bone drill to create a hole in the calvaria (ensure that the drill specifications match those of the ground screw). Secure the ground screw and attach the EIB to the skull using dental cement.
NOTE: Small amounts of hydrogen peroxide can be applied to increase the porosity of the skull and enhance the adherence of the dental cement. - Having identified the VL muscle, implant the custom-fabricated epimysial electrode implanted in line with the muscle fibers using 4-0 nonabsorbable monofilament. Close the incision using wound clips or by suturing.
- Following closing, remove the animal from anesthesia and house individually in a clean, dry animal cage. Allow the rat to recover on a heated pad and monitor the rat's temperature and respiratory rate every 15 minutes until the animal is ambulatory. Treat post-surgical pain by administering an approved analgesic in the days following instrumentation.
NOTE: rat needs to be housed individually until fully recovered. - After a 14 day period, perform in vivo electrode testing to assess the validity of the custom-fabricated epimysial electrode to capture physiological alterations in muscle activity. Place the instrumented rat on a rodent treadmill and randomly expose it to uphill and downhill walking conditions that elicit an increase or reduction in VL EMG signaling (16° incline, 16° decline at 16 meters/minute). Collect longitudinal data (e.g., 14 days and 56 days post instrumentation) to ensure reliability over time.
NOTE: 14- and 56-day assessments have been selected as a period of 14 days allows for complete surgical recovery and that of 56 days exceeds our experimental timeline. We encourage others to test electrode performance and reliability using a timeframe that replicates their experimental timeline before use. Speed (16m/min) and walking conditions (16° incline, 16° decline) were selected to match established conditions of increased and decreased VL excitation in this model10. - Modify the acquisition parameters to suit the experimental needs. Capture EMG signaling during walking at a rate of 2,000 Hz using the data acquisition platform and synchronize with a motion capture system.
NOTE: If using a different acquisition device, collect EMG and motion capture according to the manufacturer's instructions and to meet experimental needs. - Extract 25 s of gait data from each walking condition and export to a customized Python script for further signal processing and analysis. Baseline-adjust (zero), rectify, and smooth using a root mean square algorithm with a 50 ms bin width. Apply a peak detection algorithm to select RMS-EMG peaks to utilize for subsequent data analyses.
NOTE: We have briefly described methods to pre-process and analyze EMG data, as this step varies greatly due to programming languages and EMG acquisition software and hardware. We encourage authors to consult the following resources for additional information3,11,12,13.
4. Biocompatibility testing
- Following the end of longitudinal EMG collections (or at a selected time point), euthanize the rat using appropriate and approved methods (e.g., asphyxiation using CO2 followed by bilateral thoracotomy).
- Following euthanasia, extract bilateral VL muscles by creating an incision on the anterolateral right and left hindlimbs. Flash freeze in liquid nitrogen and store at -80 °C.
NOTE: Take care to keep the electrode in position on the instrumented muscle (e.g., the right VL) during extraction. Alternatively, mark the sub-electrode region or extract only the muscle from the sub-electrode region. This does not apply to the contralateral/control muscle. - Use a cryostat to cut serial muscle cross-sections (7 µm) from the region directly underneath the electrode-fascial interface. Cut sections from the muscle belly of the contralateral VL to serve as a control. See Kumar et al. for details on cryosectioning14.
- Stain with Hematoxylin & Eosin (H&E); see Wang et al. for details regarding H&E staining on skeletal muscle cross sections15.
- Examine the stained sections (instrumented and control) using a light microscope at 20x magnification. Analyze histological changes blind to the origin of the samples. Assess tissue health assessed using the following pathological features: immune cell infiltration, internal myonuclear accumulation, fibrogenesis, and sarcolemma fragmentation.
5. Suggested statistical analyses
- To assess the performance of the custom-fabricated epimyisal electrode in comparison to the industry standard, import the ex vivo data to a selected statistical package. Apply the following statistical tests to the ex vivo data collected from the custom-fabricated and industry standard electrodes (e.g., 8,000 representative samples from the sine, triangle, and square waveforms). Set alpha level a priori at p ≤ 0.05.
- To test the level of signal agreement between electrodes, use intraclass correlations (ICCs) and Bland-Altman plots. Calculate ICC estimates and their 95% confidence intervals based on a single-rating (k = 2), absolute-agreement, 2-way random-effects model.
- To test the degree to which the recording of the waveforms covaried over time, also known as temporal synchrony, perform Pearson correlation on values collected with the custom-fabricated and industry standard electrodes.
- Compare the mean electrode impedance for both contact surfaces (measured at 1 kHz) from selected custom-fabricated electrodes (e.g., 10 electrodes) to the impedance of the industry-standard electrode.
NOTE: Electrode impedance values will differ based on the use case and materials used during electrode fabrication.
- To assess the quality of the in vivo recordings, import the data collected during treadmill walking to a selected statistical package.
- To assess the validity of the custom-fabricated electrode during in vivo testing (where the rat's gait was perturbed via uphill and downhill walking to induce physiological alterations in muscle activity), perform Welch's t-test to compare mean peak values for uphill and downhill walking conditions.
NOTE: Readers are encouraged to perform additional tests and analyses (e.g., signal-to-noise ratio (SNR) assessments; see Delysys Fundamental Concepts in EMG Signal Acquisition) that are not presented here for brevity16. - To assess the reliability of the electrode's signaling over time, evaluate the longitudinal data collected during consistent conditions (level walking at 16 m/min at 14 and 56 day intervals post implantation) via paired t-tests.
- To assess the validity of the custom-fabricated electrode during in vivo testing (where the rat's gait was perturbed via uphill and downhill walking to induce physiological alterations in muscle activity), perform Welch's t-test to compare mean peak values for uphill and downhill walking conditions.
Results
Ex vivo performance
ICCs revealed high levels of agreement between the custom-fabricated and industry standard electrodes across all waveforms (sine [ICC = 0.993], square [ICC = 0.995], triangle [ICC = 0.958]; p < .001). Bland-Altman plots also revealed a high degree of signal agreement between electrodes. Bland Altman plots and Pearson correlations are summarized in Figure 3 with strong positive correlations between the custom-fabricated and industry stan...
Discussion
Our objective was to streamline the EMG fabrication process, enabling broader adoption and implementation of epimysial electrode designs, thus promoting accessibility, and advancing neuromuscular research. To this end, we present a user-friendly guide for sourcing, fabricating, and testing low-cost epimysial electrodes in-house. In hopes of supporting other research groups, we also provide supplemental 3D printing templates to facilitate the production of in-house epimysial electrodes for their research endeavors.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01AR081235 (to L. K. Lepley). The authors thank the following individuals for their contribution to the fabrication and testing of our biocompatible electrode: Joel Pingel, Grant Gueller, Akhil Ramesh, Joe Letner, Jacky Tian, and Ross Brancati.
Materials
| Name | Company | Catalog Number | Comments |
| Electrode Materials | |||
| Quantity & price per electrode | |||
| Contact surface | Prince and Izant PT90/IR10 1.25 mm x 5 mm foil | Catalog #1040055 | 2 per electrode $7.50 per foil $15.00 per electrode |
| PFA coated stainless-steel electrode lead wire | A-M Systems Multi-Stranded PFA-Coated Stainless Steel Wire 50.8 µm strand diameter | Catalog #793500 | Dependent on desired lead length (e.g., 9 inch lead wires x2) $128 per 25 ft spool $5.12 per foot $0.42 per inch (x18) $7.68 per electrode |
| Folding jig | 3D printed (see .gcode file) | NA | NA |
| Sealant for electrode body | Nusil Med-1137 liquid silicone | Catalog #MED-1137 | 1 gram $344.66 per 2 oz. (59.15 mL) $5.83 per electrode |
| Silicone base | Implantech Alliedsil Silicone Sheeting-Reinforced, Long Term Implantable (8” x 6”) .007 thick | Catalog #701-07 | 10mm x 5mm sheet $225.00 per 8 x 6 inch $0.36 per electrode (10 mm x 5 mm) |
| Thinner for sealant mixture | Toluene 99.5% ACS Reagent 500mL or Xylene ACS 99.5% | Catalog #179418-500 ML | 0.75 mL $25.53 per 500 mL $0.38 per electrode |
| Template for perforating silicone base | Cutting jig – 3D printed (see CAD file) | NA | NA |
| Custom-fabricated electrode: $29.25 | |||
| Industry standard electrode (EP105 EMG Patch Electrode, 2 contacts, single-sided, 7mm x 4mm, MicroProbe for Life Science): $305.00 | |||
| Additional Fabrication Materials | |||
| Quantity & price per electrode | |||
| 3D printing software | Solidworks (Solidworks, 2022) | ||
| Micro-Tig welder | Micro-Tig Welder (CD1000SPM, Single Pulse Research and Light Production Resistance Spot Welder, Sunstone) | SKU 301010 | $3,500 |
| Ultrasonic bath | Ultrasonic bath (CPX Series Ultrasonic Bath, Fisherbrand). | 15-337-403 | NA |
| Ex Vivo Testing Materials | |||
| Quantity & price per electrode | |||
| Data acquisition platform and software | DigitalLynx 4sX Base Cheetah version 6.0 (Neuralynx Inc.) | NA | EMG acquisition hardware and software |
| Electrode interface board (EIB) | EIB, EIB16-QC, Neuralynx Inc. | 31-0603-0007 | NA |
| Signal generator | 5 MHz Function Generator, B&K Precision | 4005DDS220V | $387.46 |
| Potentiostat | PGSTAT1 potentiostat (EcoChemie, Utrecht, Netherlands) | NA | NA |
| Stainless steel screw | Fine Science Tools | 19010-00 | $98 |
| Ex Vivo Testing Materials | |||
| Quantity & price per electrode | |||
| Rodent treadmill | Exer 3/6 Open Treadmill, Columbus Instruments | NA | NA |
| Dental cement | Excel Formula® Pourable Dental Material, St. George Technology Inc. | #24211 | $125.60 |
| Light microscope | Keyence BZ-X800, Keyence Corporation, Osaka, Japan | NA | NA |
| Motion capture system | Optitrack Color Camera, Optitrack, NaturalPoint Inc. | NA | NA |
| Peak detection algorithm | “SciPy.signal.find_peaks - SciPy v1.8.1 Manual”, 2022 | NA | NA |
| Python software | Python Software Foundation. Python Language Reference, version 3.9. Available at http://www.python.org | NA | NA |
| Rat | HsdBlu: LE, Envigo | 140 | NA |
| Statistical sotware | GraphPad Prism version 10.0.0 (GraphPad Software, Boston, Massachusetts USA) | NA | NA |
References
- Grandjean, P. A., Mortimer, J. T. Recruitment properties of monopolar and bipolar epimysial electrodes. Ann. Biomed. Eng. 14 (1), 53-66 (1986).
- Memberg, W. D., Stage, T. G., Kirsch, R. F. A fully implanted intramuscular bipolar myoelectric signal recording electrode. Neuromodulation J. Int. Neuromodulation Soc. 17 (8), 794-799 (2014).
- Loeb, G. E., Gans, C. . Electromyography for Experimentalists. , (1986).
- Boehler, C., Carli, S., Fadiga, L., Stieglitz, T., Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15 (11), 3557-3578 (2020).
- Patel, P. R., et al. Insertion of linear 8.4 µm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 12 (4), 046009 (2015).
- Richie, J. M., et al. Open-source toolkit: benchtop carbon fiber microelectrode array for nerve recording. J. Vis. Exp. (176), e63099 (2021).
- Sarolic, A., Skalic, I., Deftu, A., Sapunar, D. Impedance measurement of bipolar stimulation electrodes immersed in medium. , 1-2 (2018).
- Pritchett-Corning, K. R., Mulder, G. B., Luo, Y., White, W. J. Principles of rodent surgery for the New Surgeon. J. Vis. Exp.: JoVE. (47), e2586 (2011).
- Zealear, D., Li, Y., Huang, S. An implantable system for chronic in vivo electromyography. J. Vis. Exp. JoVE. (158), e60345 (2020).
- Butterfield, T. A., Leonard, T. R., Herzog, W. Differential serial sarcomere number adaptations in knee extensor muscles of rats is contraction type dependent. J. Appl. Physiol. Bethesda Md 1985. 99 (4), 1352-1358 (2005).
- Farago, E., MacIsaac, D., Suk, M., Chan, A. D. C. A review of techniques for surface electromyography signal quality analysis. IEEE Rev. Biomed. Eng. 16, 472-486 (2023).
- Raez, M. B. I., Hussain, M. S., Mohd-Yasin, F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proced. Online. 8, 11-35 (2006).
- Tankisi, H., et al. Standards of instrumentation of EMG. Clin. Neurophysiol. 131 (1), 243-258 (2020).
- Kumar, A., Accorsi, A., Younghwa, R., Mahasweta, G. Do's and don'ts in the preparation of muscle cryosections for histological analysis. J. Vis. Exp. JoVE. (99), e52793 (2015).
- Wang, C., Yue, F., Kuang, S. Muscle histology characterization using H&E staining and muscle fiber type classification using immunofluorescence staining. Bio-Protoc. 7 (10), e2279 (2017).
- Kreifeldt, J. G. Signal versus noise characteristics of filtered EMG used as a control source. IEEE Trans. Biomed. Eng. BME-18 (1), 16-22 (1971).
- Farina, D., Yoshida, K., Stieglitz, T., Koch, K. P. Multichannel thin-film electrode for intramuscular electromyographic recordings. J. Appl. Physiol. 104 (3), 821-827 (2008).
- Muceli, S., et al. Decoding motor neuron activity from epimysial thin-film electrode recordings following targeted muscle reinnervation. J. Neural Eng. 16 (1), 016010 (2018).
- Guo, L., Guvanasen, G., Tuthill, C., Nichols, T., Deweerth, S. . Characterization of a Stretchable Multielectrode Array for Epimysial Recording. , 694 (2011).
- Zwarts, M. J., Stegeman, D. F. Multichannel surface EMG: basic aspects and clinical utility. Muscle Nerve. 28 (1), 1-17 (2003).
- Koch, K. P., Leinenbach, C., Stieglitz, T. Fabrication and test of robust spherical epimysial electrodes for lower limb stimulation. Aalb. Den. , (2000).
- Uhlir, J. P., Triolo, R. J., Davis, J. A., Bieri, C. Performance of epimysial stimulating electrodes in the lower extremities of individuals with spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 12 (2), 279-287 (2004).
- Deer, T. R., et al. The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Technol. Neural Interface. 17 (6), 599-615 (2014).
- Ortiz-Catalan, M., Brånemark, R., Håkansson, B., Delbeke, J. On the viability of implantable electrodes for the natural control of artificial limbs: Review and discussion. Biomed. Eng. OnLine. 11, 33 (2012).
- Sando, I. C., et al. Regenerative peripheral nerve interface for prostheses control: electrode comparison. J. Reconstr. Microsurg. 32 (3), 194-199 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved