A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Establishment of an Embryo Implantation Model In Vitro
In This Article
Summary
This protocol describes methodologies to establish simple and efficient embryo implantation in vitro model for evaluating the relevant molecules affecting the embryo implantation process.
Abstract
Embryo implantation is the first step in the establishment of a successful pregnancy. An in vitro model for embryo implantation is critical for basic biological research, drug development, and screening. This paper presents a simple, rapid, and highly efficient in vitro model for embryo implantation. In this protocol, we first introduce mouse blastocyst acquisition and human endometrial adenocarcinoma cells (Ishikawa) preparation for implantation, followed by the co-culture method for mouse embryos and Ishikawa cells. Finally, we conducted a study to assess the impact of varying concentrations of 17-β-estradiol (E2) and progesterone (P4) on embryo adhesion rates based on this model. Our findings revealed that high concentrations of E2 significantly reduced embryo adhesion, whereas the addition of progesterone could restore the adhesion rate. This model offers a simple and fast platform for evaluating and screening molecules involved in the adhesion process, such as cytokines, drugs, and transcription factors controlling implantation and endometrial receptivity.
Introduction
Embryo implantation, the initial step of successful pregnancy, is crucial for understanding its biological basis and addressing the challenges of infertility. However, ethical constraints pose significant limitations in collecting human clinical embryo samples, hampering research into the intricate interactions between human embryos and the endometrium during early pregnancy1. A profound comprehension of these complex mechanisms is vital for advancing fundamental biological research, drug discovery, and reproductive health.
Previous in vitro models employed adhesion models where monolayer human endometrial epithelial cells (RL95-2)2 were co-cultured with embryonic substitutes, such as BeWo, JAr, or Jeg-33 choriocarcinoma cells. Nevertheless, the selection of the size of the spheroids, ranging from 70-100 µm, was crucial as larger or smaller cell aggregates could compromise the accuracy of the experiment. Notably, only 25% of the spheroids in each preparation met the standard for the suitable size4, and there were significant differences compared to the physiological conditions of blastocysts.
Furthermore, three-dimensional (3D) culture systems for the endometrium and blastocyst offer a more physiologically relevant environment5, but they require high technical expertise, slow cell growth, high resource and maintenance costs, difficulty in standardization, and low reproducibility6.
In this protocol, we present a cost-effective and rapid in vitro model of embryo implantation that can be developed and utilized in most laboratories. The overall objective of this method is to provide a reliable and reproducible platform for studying the interactions between embryos and the endometrium in a controlled environment. By simulating key events of embryo implantation in vitro, this model aims to bridge the gap between animal models and clinical settings, enabling more precise and targeted research.
Compared to embryonic substitutes, the use of mouse blastocysts offers a more physiologically relevant representation of the in vivo embryo implantation process7. In addition, the Ishikawa cell line, derived from human endometrial adenocarcinoma, possesses mixed characteristics of glandular epithelium and uterine lumen epithelium1, enabling it to express enzymes, structural proteins, and functional steroid receptors similar to those found in normal endometrium, thus providing a physiologically relevant substrate for embryo implantation. The simplicity and rapidity of the co-culture system enable high-throughput screening and drug testing8,9,10. By providing a robust and reproducible platform, this method offers an opportunity to advance our understanding of embryo implantation and its impact on human health.
Protocol
Mouse handling and experimental studies were performed under protocols approved by the Animal Committee of the Shanghai Institute for Biomedical and Pharmaceutical Technologies. Ensure Safety Procedures: Always wear appropriate personal protective equipment (PPE) when handling chemicals or biological materials.
1. Acquisition of mouse embryos
NOTE: This section describes the process of obtaining mouse embryos. Adult female mice (6-8 weeks old, hybrid C57BL/6 and DBA/2) were maintained under standard environmental conditions of 12 h light and 12 h dark at 20-25 °C and 40-60% humidity with food and water provided ad libitum.
- Inject female mice intraperitoneally with 10 IU of pregnant mare's serum gonadotropin (PMSG) and then, injected with 10 IU of human chorionic gonadotropin (HCG) at a 42-48 h interval.
- Mate each female mouse with a male of comparable age and check for a vaginal plug the next morning.
NOTE: Vaginal plug is a light yellow lump of suppository formed after semen solidifies at the vaginal opening. - Euthanize the female mice with vaginal plugs by CO2 inhalation.
- Open the abdomen and locate the female genitalia; stretch the oviduct, ovary, and fat pads with forceps; cut the oviduct and ovary with scissors; and then, cut the oviduct and uterus (Figure 1).
- Transfer the obtained oviduct to a 35 mm Petri dish prefilled with M2 medium.
NOTE: M2 medium should be preheated in an incubator (37 °C, 5% CO2) for 30 min. - Puncture the enlarged ampulla of the oviduct with a 25 G needle to release or pinch the cumulus-zygote complex (Figure 2).
- Use forceps to transfer the cumulus-zygote complex to M2 solution containing 0.5 mg/mL hyaluronidase and incubate for several minutes until the cumulus cells dissociate (Figure 3).
- Collect the zygotes using an egg transfer pipette and wash them in M2 medium containing 4 mg/mL BSA to remove residual hyaluronidase, cumulus cells, and impurities.
2. In vitro culture of mouse embryo
NOTE: This section details the process of in vitro culturing mouse embryos from zygotes to blastocysts. KSOM culture microdrops should be prepared 24 h ahead to ensure temperature and gas equilibrium.
- Prepare 12 microdroplets of KSOM medium, each with a volume of 20 µL, at the bottom of a 35 mm Petri dish. Cover the microdroplets with 3-5 mL of mineral oil (Figure 4), and place the Petri dishes in an incubator at 37 °C with 5% CO2 for equilibration.
- Transfer the mouse embryos from M2 medium to KSOM medium using a transfer pipette for thorough washing.
NOTE: This step requires a stereomicroscope with a 37 °C heating stage. Minimize the duration of embryo handling outside the incubator. - Place the washed embryos in the prepared KSOM microdroplets and incubate them at 37 °C with 5% CO2 for 4 days.
NOTE: To ensure adequate nutrition, culture 5-10 embryos per 20 µL microdrop. - Select advantageous blastocysts according to their size (100-130 µm), inner cell mass (the inner cell mass is composed of many cells that are all compacted), number of trophoblast cells (the trophectodermis composed of many cells that are cohesive and packed together), and the degree of expansion (blastocoel occupies 80-100% of the embryo).
3. Preparation of endometrial cells
- Inside a biosafety cabinet, aspirate 1 mL of 2% gelatin using a pipette and dispense it into a 12-well culture plate. Gently rotate the plate to ensure complete coverage of the bottom with the gelatin. Place the plate in a 37 °C incubator for 30 min. Afterward, aspirate the excess gelatin and allow the bottom of the plate to dry completely.
- After the coating process is complete, culture human endometrial adenocarcinoma cells (Ishikawa) with DMEM/F12 (see the Table of Materials for details), 1 mM sodium pyruvate, 2 mM L-glutamine, 100 µg/mL streptomycin, and 100 IU/mL penicillin, seed them into the gelatin-coated 12-well plates (2 × 105cells/well), and incubate for 24 h.
4. Embryo attachment
- Gently place 5-15 fresh blastocysts into each well of the 12-well plate containing Ishikawa cells prepared in the previous step for co-culture (Figure 5).
- After 48 h incubation, observe the embryos under a microscope to assess their attachment status. To observe embryo attachment, quickly move the plate three times. Unattached embryos will exhibit floating or rolling motion over the surface of the Ishikawa cells.
- Calculate the embryo implantation rate using equation (1); use the embryo adhesion rate to represent the implantation effect.
Embryo Implantation Rate = (Attached Embryos/Total Number of Implanted Embryos) × 100% (1)
Results
In the process of assisted reproductive techniques, controlled ovarian hyperstimulation (COH) is a crucial step, leading to significantly elevated estrogen levels in patients by more than 10-20 times compared to natural cycles11. Given this context, we asked whether high serum estrogen concentrations impact embryo implantation during fresh embryo transfer. Based on this embryo implantation model, we studied the effects of various concentrations of E2 and P4 on embryo implantat...
Discussion
The simplicity and rapidity of the proposed in vitro model for embryo implantation offer remarkable advantages for early-stage drug screening and other research applications. The straightforward protocol, coupled with the high-throughput nature of Ishikawa cell lines, makes it an ideal candidate for large-scale screens, particularly in the early stages of drug discovery. Liang13 found that TAGLN2 knockdown decreased the invasion ability of trophoblast cells. Green14
Disclosures
The authors have no conflicts of financial or other interests to declare.
Acknowledgements
Our studies were supported by the National Natural Science Foundation of China (82171603), the Foundation of Shanghai Municipal Health Commission (202140341), the Science and Technology Commission of Shanghai Municipality (23JC1403803), and Innovation Promotion Program of NHC and Shanghai Key Labs, SIBPT (RC2023-02).
Materials
| Name | Company | Catalog Number | Comments |
| 17-β-estradiol | SIGMA | 3301 | Most potent mammalian estrogenic hormone |
| BD Falcon | BD | 353001 | Bacteriological Petri Dishes 35 x 10 mm style w/tight lid, crystal-grade virgin polystyrene, sterile |
| Biosafety Cabinet | ESCO | class 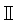 BSC BSC | Aseptic operations, making culture dishes, aliquoting reagents, etc |
| CO2 Incubator | Thermo | 8000DH | Embryo culture |
| Culture plate | Corning | 3506 | Cell culture |
| DMEM/F12 | Gibco | 1133032 | DMEM/F12 (1x), liquid 1:1,Contains L-glutamine and 15 mM HEPES buffer |
| Fetal Bovine Serum | Gibco | 10099141 | Fetal Bovine Serum, Qualified, Australia Origin |
| Gelatin | SIGMA | G9391 | Type B, powder, BioReagent, suitable for cell culture |
| HCG | Nanjing Aibei | M2520 | Sterilization reagent, intraperitoneal injection, 50 IU/mL |
| Hyaluronidase | SIGMA | V900833 | Reagent grade, powder |
| KSOM | Merck | MR-020P-D | (1x), Powder, w/o Phenol Red, 5 x 10 mL |
| L-glutamine | Gibco | 25030081 | L-glutamine-200 mM (100x), liquid |
| M2 | Merck | MR-015-D | EmbryoMax M2 Medium (1x), Liquid, with Phenol Red |
| Mineral oil | SIGMA | M8410 | Mineral oil is suitable for use as a cover layer to control evaporation and cross-contamination in various molecular biology applications |
| Penicillin-Streptomycin, liquid | Gibco | 15140122 | 10,000 Units penicillin (base) and 10,000 units streptomycin (base), utilizing penicillin G (sodium salt) and streptomycin sulfate in 0.85% saline |
| PMSG | Nanjing Aibei | M2620 | Sterilization reagent ,intraperitoneal injection, 50 IU/mL |
| Progesterone | SIGMA | 5341 | Steroid hormone secreted by the corpus luteum during the latter half of the menstrual cycle |
| Sodium pyruvate | Gibco | 11360070 | Sodium pyruvate is commonly added to cell culture media as a carbon source in addition to glucose |
| Stereomicroscope | Olympus | SZX7 | Embryo retrieval and observation of embryo development |
References
- Hannan, N. J., Paiva, P., Dimitriadis, E., Salamonsen, L. A. Models for study of human embryo implantation: choice of cell lines. Biol Reprod. 82 (2), 235-245 (2010).
- Li, H. Y., et al. Establishment of an efficient method to quantify embryo attachment to endometrial epithelial cell monolayers. In Vitro Cell Dev Biol Anim. 38 (9), 505-511 (2002).
- Hohn, H. P., Linke, M., Denker, H. W. Adhesion of trophoblast to uterine epithelium as related to the state of trophoblast differentiation: in vitro studies using cell lines. Mol Reprod Dev. 57 (2), 135-145 (2000).
- Ho, H., Singh, H., Aljofan, M., Nie, G. A high-throughput in vitro model of human embryo attachment. Fertil Steril. 97 (4), 974-978 (2012).
- Karvas, R. M., et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell. 29 (5), 810-825 (2022).
- Xiang, L., et al. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature. 577 (7791), 537-542 (2020).
- Ojosnegros, S., Seriola, A., Godeau, A. L., Veiga, A. Embryo implantation in the laboratory: an update on current techniques. Hum Reprod Update. 27 (3), 501-530 (2021).
- Berneau, S. C., et al. Characterisation of osteopontin in an in vitro model of embryo implantation. Cells. 8 (5), 432 (2019).
- Jiang, R., et al. Enhanced HOXA10 sumoylation inhibits embryo implantation in women with recurrent implantation failure. Cell Death Discov. 3, 17057 (2017).
- Kang, Y. J., Forbes, K., Carver, J., Aplin, J. D. The role of the osteopontin-integrin αvβ3 interaction at implantation: functional analysis using three different in vitro models. Hum Reprod. 29 (4), 739-749 (2014).
- Duan, C. C., et al. Oocyte exposure to supraphysiological estradiol during ovarian stimulation increased the risk of adverse perinatal outcomes after frozen-thawed embryo transfer: a retrospective cohort study. J Dev Orig Health Dis. 11 (4), 392-402 (2020).
- Chen, G., et al. Integrins β1 and β3 are biomarkers of uterine condition for embryo transfer. J Transl Med. 14 (1), 303-312 (2016).
- Liang, X., et al. Transgelin 2 is required for embryo implantation by promoting actin polymerization. FASEB J. 33 (4), 5667-5675 (2019).
- Green, C. J., Fraser, S. T., Day, M. L. Insulin-like growth factor 1 increases apical fibronectin in blastocysts to increase blastocyst attachment to endometrial epithelial cells in vitro. Hum Reprod. 30 (2), 284-298 (2015).
- Kang, Y. J., et al. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J Cell Sci. 128 (4), 804-814 (2015).
- Berger, C., Boggavarapu, N. R., Menezes, J., Lalitkumar, P. G., Gemzell-Danielsson, K. Effects of ulipristal acetate on human embryo attachment and endometrial cell gene expression in an in vitro co-culture system. Hum Reprod. 30 (4), 800-811 (2015).
- Li, X., et al. Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation. Reprod Biol Endocrinol. 20 (1), 120-138 (2022).
- Fitzgerald, H. C., Schust, D. J., Spencer, T. E. In vitro models of the human endometrium: evolution and application for women's health. Biol Reprod. 104 (2), 282-293 (2021).
- Ahn, J., et al. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum Reprod. 36 (10), 2720-2731 (2021).
- Sternberg, A. K., Buck, V. U., Classen-Linke, I., Leube, R. E. How mechanical forces change the human endometrium during the menstrual cycle in preparation for embryo implantation. Cells. 10 (8), 2008 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved