A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
A Modified Model Preparation for Middle Cerebral Artery Occlusion Reperfusion
In This Article
Summary
This protocol describes the process of preparing for middle cerebral artery occlusion reperfusion via the common carotid artery.
Abstract
The middle cerebral artery occlusion reperfusion (MCAO/R) model is crucial for understanding the pathological mechanisms of stroke and for drug development.However, among the commonly used modeling methods, the Koizumi method often faces scrutiny due to its ligation of the common carotid artery (CCA) and its inability to achieve adequate reperfusion. Similarly, the Longa method has been criticized for disconnecting and ligating the external carotid artery (ECA). This study aims to introduce a modified model preparation method that preserves the integrity of the ECA, involves inserting a monofilament nylon suture through the CCA, repairing the ligated CCA incision, and maintaining reperfusion from the CCA. Reperfusion of blood flow was confirmed using laser speckle flow imaging. Evaluation methods such as the Longa scale, Modified Neurological Severity Score, triphenyltetrazolium chloride (TTC) staining, and immunofluorescence labeling of neurons demonstrated that this approach could induce stable ischemic nerve damage. This modified MCAO/R model protocol is simple and stable, providing valuable guidance for practitioners in the field of cerebral ischemia.
Introduction
According to the World Health Organization, stroke has remained the second leading cause of death worldwide for the past decade, with a high incidence rate, high mortality, and high disability rate1,2. As the global population ages, the incidence of stroke is expected to increase in developing countries, potentially becoming the leading cause of premature death and disability in adults. Additionally, there is a trend for strokes to occur at a younger age3. The loss of the labor force after a stroke also places a heavy burden on families and society4. Therefore, the development of safe and effective treatments poses a major challenge in stroke research.
Animal models serve as crucial tools for studying the prevention and treatment of human diseases. The successful translation of stroke treatment strategies relies on the reproducibility and reliability of stroke animal models5,6. The middle cerebral artery (MCA) is a common site for clinical stroke, making the MCAO model the closest model to human ischemic stroke. The MCAO model, prepared using the suture method, has been favored by researchers due to advantages such as no craniotomy and easy control of ischemic time. It has been utilized in over 40% of neuroprotective experiments7. However, despite its numerous advantages, the operational details of this model remain a controversial topic for many researchers.
For the suture-induced middle cerebral artery occlusion (MCAO) model, reperfusion occurs by withdrawing the suture. Currently, two main methods are used for suture insertion: Koizumi's method8 and Longa's method9. In Koizumi's method, the suture enters the internal carotid artery (ICA) mainly through the common carotid artery (CCA) incision, while in Longa's method, it passes through the severed external carotid artery (ECA) into the ICA. During reperfusion, the Koizumi method requires permanently ligating the CCA incision and relies on the circle of Willis for reperfusion10. However, some studies suggest that effective reperfusion cannot be achieved solely through the compensatory supply of the circle of Willis after losing the CCA supply. Moreover, the circle of Willis exhibits high anatomical variability, especially in C57Bl/6 mice, increasing infarction variability and reducing experimental data reliability. Consequently, this method has been increasingly questioned by researchers11.
Longa's method involves inserting a suture through the severed ECA and then permanently ligating the internal carotid artery (ICA) once the suture is withdrawn. This preserves CCA patency, allowing blood perfusion up to 100% of baseline values. However, this method necessitates separating the external carotid artery and small arterial branches, cutting them off, or electrocoagulating them, making the procedure challenging. It also disrupts the brain's complete blood flow structure, which differs from the clinical patient's state12. Importantly, studies indicate that cutting or ligating the ECA can cause ischemic lesions in the muscles controlling chewing and swallowing, affecting animal diet and leading to postoperative animal death and severe sensory and motor damage in rats13,14.
Hence, a modified model preparation method is urgently needed to address these issues. This study introduces a modified MCAO modeling method that repairs the CCA insertion incision and achieves effective reperfusion. The procedure is simple, practical, and feasible, inducing significant neurological damage and replicable infarct lesions and providing valuable guidance for stroke researchers.
Protocol
The experimental protocol was conducted in compliance with the Use of Laboratory Animals and Institutional Animal Care and Use Committee guidelines at Chengdu University of Traditional Chinese Medicine (Record number: 2019-DL-002). All animal research data have been documented following the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Male Sprague Dawley (SD) rats weighing 250 g ± 20 g and aged 6-8 weeks were utilized for this study. The specifics regarding the animals, reagents, and equipment employed are listed in the Table of Materials.
1. Animal preparation
- Induce and maintain deep anesthesia in rats using Zoletil 50 (50mg/kg, IM) and Xylazine Hydrochloride (40mg/kg, IP). Ensure the body temperature is maintained at 37 ± 0.5 °C using a rectal probe connected to a heating pad throughout the surgical procedure. Apply veterinary ointment to the rat's eyes to prevent them from drying out.
- Shave the hair from the head and neck of the rats. Use depilatory cream to remove fur from the head and neck, then wash off the cream with normal saline. Disinfect the skin at the surgical site by applying ethanol and povidone-iodine three times using sterile cotton balls.
- Make a 2 cm incision in the middle of the rat's head along the direction of the sagittal suture using a scalpel, and carefully remove the muscles covering the skull.
- Thin the skull on the ischemic side of the rat using a skull drill. Use normal saline to cool down and remove debris during the grinding process. Record the baseline blood flow of the rats using laser speckle contrast imaging (LSCI).
- Apply ethanol and povidone-iodine three times to the skin of the neck using sterile cotton balls. Make a 2 cm incision along the midline of the neck using a surgical blade. Use retractors to laterally pull back the skin and salivary glands, exposing the sternocleidomastoid and cervical muscles.
- Separate the sternocleidomastoid and cervical muscles to expose the carotid territory. Identify the vascular anatomy of the common carotid artery (CCA), the internal carotid artery (ICA), and the external carotid artery (ECA). Based on vascular anatomy, separate the CCA, as well as the CCA-derived ECA and ICA15.
NOTE: The anatomical structure of the rat indicates that the CCA region is mainly covered by sternocleidomastoid and cervical muscles, with the ICA and ECA derived from the CCA. After separation exposes the CCA, a Y-shaped fork can be seen along the CCA, representing the ICA and ECA. Be cautious not to damage the vagus nerve, which runs parallel to the CCA.
2. Occlusion of the MCA
- Tie an easily untangled knot over the external carotid artery (ECA) and common carotid artery (CCA) using a 3-0 silk thread to temporarily block blood flow. Position a vessel clip on the CCA approximately 0.5 cm from the first knot.
- Dye the needle of a 5 mL syringe black. Create a small puncture in the CCA using the black-dyed needle and mark the pinholes in black.
- Insert the monofilament nylon suture into the CCA through the black mark. Open the vascular clip and guide the nylon wire into the Internal Carotid Artery (ICA) until it halts with slight resistance. Tighten the second knot securely to keep the monofilament nylon suture in place inside the artery, preventing displacement from the blocking position.
- Note the time of ischemia at this point and measure the blood flow value on the ischemic side using LSCI. Administer drops of bupivacaine to the neck wound for analgesia and wound closure. Remove the anesthesia mask and allow the rat to recover.
NOTE: If the monofilament nylon suture encounters difficulty entering the ICA, retract it slightly and attempt insertion again.
3. Reperfusion of MCAO
- Following 90 min of ischemia, re-anesthetize the rats with isoflurane. Measure cerebral blood flow from the ischemic side using LSCI and ensure the monofilament nylon suture has not shifted.
- Untie the knots on the external carotid artery (ECA) to allow blood reperfusion. Release the knot on the common carotid artery (CCA), secure the monofilament suture, and withdraw the suture. Apply a vascular clip ahead of the fixed monofilament suture to prevent bleeding.
- Replace the first CCA knot with a vascular clip. Use tweezers to rotate the vessel incision sideways (Figure 1). Clamp the incision with tweezers and ligate it using 6-0 thread to repair the incision. Remove the vascular clip, check for leaks, and confirm complete reperfusion (Video 1).
NOTE: Perform ligation to repair the incision to avoid excessive vessel clamping, which could lead to CCA stenosis. - Record cerebral cortical blood flow values using LSCI after incision ligation to confirm successful reperfusion. Close the neck incision with sutures and administer 1,00,000 units of penicillin and 2 mL of saline to prevent infection and dehydration.
- Maintain warmth until the rats regain consciousness. Offer soft food post-surgery and monitor the animals' vital signs.
4. Evaluation of nerve function and cerebral ischemic injury
- Once the rats have fully awakened, their neural function will be assessed by researchers unaware of the animal grouping. Utilize the Longa score scale (Table 1) and the modified neurological severity score (mNSS) scale (Table 2) to evaluate the neurological function of all rats.
- After 24 h post-surgery, euthanize the rats under isoflurane-induced anesthesia (4% isoflurane at 4 L/min oxygen) via cervical dislocation16 (following institutionally approved protocols). Rinse the brain with ice water to remove any remaining blood.
- Divide the brain tissue into 5 sections and stain them with TTC. Capture images of both sides of the brain tissue slices, measure the ischemic area of the front and back sides using Image J software, and calculate the infarction rate using the following formula:
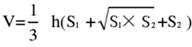
NOTE: S1 and S2 represent the infarct area of the proximal forebrain and proximal brainstem sides of the brain slices, respectively. The infarct area of each brain slice is corrected using Swanson's formula, where h represents the thickness of the brain slice. This formula, previously outlined by the author in prior research17, allows for a more accurate calculation of the infarct lesion size. - Analyze the data using statistical and graphing software to assess the model's success rate and stability.
- Employ the immunofluorescence method to label cortical neurons in rats, further validating the neuronal damage caused by this method. After 24 h of ischemia-reperfusion in rats, cardiac perfusion using pre-cooled PBS was performed to obtain brain tissue.
- Embed and freeze the brain tissue into sections. Use neuronal antibodies such as the immature neuronal marker Doublecortin (DCX)18, neuronal nucleus marker Neuronal Nuclei (NeuN)18, and neuron dendritic marker Microtubule-associated protein-2 (MAP-2)19. Observe the expression of ischemic neurons using laser confocal microscopy.
Results
Laser speckle flow imaging demonstrated that prior to the occlusion of the monofilament nylon suture, there was abundant blood flow in the middle cerebral artery (MCA) area, and the baseline blood flow values of the rats were recorded. Following the occlusion of the MCA, the blood flow value on the ischemic side of the brain rapidly decreased. Before withdrawing the suture, the blood flow values on the ischemic side were rechecked to confirm whether the suture was occluding the MCA. The results indicated only a slight ch...
Discussion
The middle cerebral artery occlusion (MCAO) model induced by a monofilament nylon suture is the most common method used for preparing MCAO models. This approach is widely adopted in preclinical studies and has gained recognition from many practitioners due to its simplicity, lack of need for craniotomy, minimal surgical trauma, and ability to achieve reperfusion.
There are two classical surgical techniques for intraluminal filament MCAO: the Koizumi method8 and the Long...
Disclosures
None.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82173781 and 82373835), Postdoctoral research project (BKS212055), Science and Technology Innovation Project of Foshan Science and Technology Bureau (2320001007331), Guangdong Basic and Applied Basic Research Foundation (2019A1515010806), Key Field Projects (Intelligent Manufacturing) of General Universities in Guangdong Province (2020ZDZX2057), and the Scientific Research Projects (Characteristic Innovation) of General Universities in Guangdong Province (2019KTSCX195).
Materials
| Name | Company | Catalog Number | Comments |
| Animal anesthesia system | Rayward Life Technology Co., Ltd | R500IE | |
| Animal temperature maintainer | Rayward Life Technology Co., Ltd | 69020 | |
| Cy3 secondary antibody | Wuhan Saiweier Biotechnology Co., Ltd | GB21303 | |
| DAP1 antibody | Wuhan Saiweier Biotechnology Co., Ltd | G1012 | |
| DCX antibody | Wuhan Saiweier Biotechnology Co., Ltd | GB13434 | |
| Goat serum | Beyotime Biotechnology Co., LTD | C0265 | |
| GraphPad Prism | GraphPad Software | GraphPad Prism 8.0 | |
| ImageJ | National Institutes of Health | ImageJ software | |
| Isofluran | Rayward Life Technology Co., Ltd | R510-22 | |
| Laser speckle blood flow imaging system | Rayward Life Technology Co., Ltd | PeriCam PSI NR | |
| MAP-2 antibody | Wuhan Saiweier Biotechnology Co., Ltd | GB11128 | |
| Miniature hand-held skull drill | Rayward Life Technology Co., Ltd | 87001 | |
| monofilament suture | Rayward Life Technology Co., Ltd | 250-280g | |
| NeuN antibody | Wuhan Saiweier Biotechnology Co., Ltd | GB11138 | |
| OCT embedding agent | BIOSHARP | BL557A | |
| Penicillin sodium | Chengdu Kelong Chemical Co., Ltd. | 17121709-2 | |
| Quick Antigen Retrieval Solution for Frozen Sections | Beyotime Biotechnology Co., LTD | P0090 | |
| SD rats | SPF ( Beijing ) Biotechnology Co.,Ltd. | 250-280g | |
| Triton X-100 | Beyotime Biotechnology Co., LTD | ST795 | |
| TTC | Chengdu Kelong Chemical Co., Ltd. | 2019030101 |
References
- Paul, S., Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp Neurol. 335, 113518 (2021).
- Feigin, V. L., Owolabi, M. O. Pragmatic solutions to reduce the global burden of stroke: a World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 22 (12), 1160-1206 (2023).
- Putaala, J. Ischemic Stroke in Young Adults. Continuum (Minneapolis, Minn). 26 (2), 386-414 (2020).
- Girotra, T., Lekoubou, A., Bishu, K. G., Ovbiagele, B. A contemporary and comprehensive analysis of the costs of stroke in the United States. J Neurol Sci. 410, 116643 (2020).
- Howells, D. W., et al. Different strokes for different folks: The rich diversity of animal models of focal cerebral ischemia. JCBFM. 30 (8), 1412-1431 (2010).
- Matur, A. V., et al. Translating animal models of ischemic stroke to the human condition. Transl Stroke Res. 14 (6), 842-853 (2023).
- O'Collins, V. E., et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 59 (3), 467-477 (2006).
- Koizumi, J., Yoshida, Y., Nakazawa, T., Ooneda, G. Experimental studies of ischemic brain edema 1. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Nosotchu. , 1-7 (1986).
- Longa, E. Z., Weinstein, P. R., Carlson, S., Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20 (1), 84-91 (1989).
- Faber, J. E., Moore, S. M., Lucitti, J. L., Aghajanian, A., Zhang, H. Sex differences in the cerebral collateral circulation. Transl Stroke Res. 8 (3), 273-283 (2017).
- Justić, H., et al. Redefining the Koizumi model of mouse cerebral ischemia: A comparative longitudinal study of cerebral and retinal ischemia in the Koizumi and Longa middle cerebral artery occlusion models. J Cereb Blood Flow Metab. 42 (11), 2080-2094 (2022).
- Li, Y., et al. Comparison of cerebral microcirculation perfusion in rat models of middle cerebral artery occlusion prepared through common carotid artery insertion and external carotid artery insertion. CJTER. 27 (11), 1683-1691 (2023).
- Dittmar, M., Spruss, T., Schuierer, G., Horn, M. External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke. 34 (9), 2252-2257 (2003).
- Trueman, R. C., et al. A critical re-examination of the intraluminal filament MCAO model: impact of external carotid artery transection. Transl Stroke Res. 2 (4), 651-661 (2011).
- Ziegler, K. A., et al. Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc Res. 114 (2), 291-299 (2018).
- Pitoulis, F. G., et al. Remodelling of adult cardiac tissue subjected to physiological and pathological mechanical load in vitro. Cardiovasc Res. 118 (3), 814-827 (2022).
- Ma, R., et al. Animal models of cerebral ischemia: A review. Biomed Pharmacother. 131, 110686 (2020).
- Belayev, L., et al. Docosanoids promote neurogenesis and angiogenesis, blood-brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol Neurobiol. 55 (8), 7090-7106 (2018).
- Guo, H., et al. Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int J Mol Med. 47 (4), 52 (2021).
- Chia, N. H., et al. Determining the number of ischemic strokes potentially eligible for endovascular thrombectomy: a population-based study. Stroke. 47 (5), 1377-1380 (2016).
- Henninger, N., Fisher, M. Extending the time window for endovascular and pharmacological reperfusion. Transl Stroke Res. 7 (4), 284-293 (2016).
- Zhang, P. L., et al. Use of Intravenous thrombolytic therapy in acute ischemic stroke patients: evaluation of clinical outcomes. Cell Biochem Biophys. 72 (1), 11-17 (2015).
- Morris, G. P., et al. A comparative study of variables influencing ischemic injury in the Longa and Koizumi methods of intraluminal filament middle cerebral artery occlusion in mice. PLOS One. 11 (2), e0148503 (2016).
- Smith, H. K., Russell, J. M., Granger, D. N., Gavins, F. N. Critical differences between two classical surgical approaches for middle cerebral artery occlusion-induced stroke in mice. J Neurosci Methods. 249, 99-105 (2015).
- Dittmar, M. S., et al. The role of ECA transection in the development of masticatory lesions in the MCAO filament model. Exp Neurol. 195 (2), 372-378 (2005).
- Lourbopoulos, A., et al. Inadequate food and water intake determine mortality following stroke in mice. J Cereb Blood Flow Metab. 37 (6), 2084-2097 (2017).
- Ogishima, H., et al. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Invest Ophth Vis Sci. 52 (13), 9710-9720 (2011).
- Irvine, H. J., et al. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cereb Blood Flow Metab. 38 (10), 1807-1817 (2018).
- Carmichael, S. T. Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx. 2 (3), 396-409 (2005).
- Dirnagl, U., Dirnagl, U. Bench to bedside: The quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 26 (12), 1465-1478 (2006).
- Ingberg, E., Dock, H., Theodorsson, E., Theodorsson, A., Ström, J. O. Method parameters' impact on mortality and variability in mouse stroke experiments: A meta-analysis. Sci Rep. 6, 21086 (2016).
- McColl, B. W., Carswell, H. V., McCulloch, J., Horsburgh, K. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res. 997 (1), 15-23 (2004).
- Kitagawa, K., et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: Evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 18 (5), 570-579 (1998).
- Trotman-Lucas, M., Kelly, M. E., Janus, J., Fern, R., Gibson, C. L. An alternative surgical approach reduces variability following filament induction of experimental stroke in mice. Dis Model Mech. 10 (7), 931-938 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved