A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Fabrication and Characterization of Microneedle Patches for Loading and Delivery of Exosomes
In This Article
Summary
Exosomes possess significant clinical potential, but their practical application is limited due to easy in vivo clearance and poor stability. Microneedles present a solution by enabling localized delivery by puncturing physiological barriers and dry-state preservation, thereby addressing the limitations of exosome administration and expanding their clinical utility.
Abstract
Exosomes, as emerging "next-generation" biotherapeutics and drug delivery vectors, hold immense potential in diverse biomedical fields, ranging from drug delivery and regenerative medicine to disease diagnosis and tumor immunotherapy. However, the rapid clearance by traditional bolus injection and poor stability of exosomes restrict their clinical application. Microneedles serve as a solution that prolongs the residence time of exosomes at the administration site, thereby maintaining the drug concentration and facilitating sustained therapeutic effects. In addition, microneedles also possess the ability to maintain the stability of bioactive substances. Therefore, we introduce a microneedle patch for loading and delivering exosomes and share the methods, including isolation of exosomes, fabrication, and characterization of exosome-loaded microneedle patches. The microneedle patches were fabricated using trehalose and hyaluronic acid as the tip materials and polyvinylpyrrolidone as the backing material through a two-step casting method. The microneedles demonstrated robust mechanical strength, with tips able to withstand 2 N. Pig skin was used to simulate human skin, and the tips of microneedles completely melted within 60 s after skin puncture. The exosomes released from the microneedles exhibited morphology, particle size, marker proteins, and biological functions comparable to those of fresh exosomes, enabling dendritic cells uptake and promoting their maturation.
Introduction
Exosomes, which are small vesicles released by cells into the extracellular matrix, have been proposed as potential biotherapeutics and drug delivery vectors for the treatment of several diseases and cancers1. During their biogenesis process, exosomes encapsulate various biologically active molecules from within the cells, including functional proteins and nucleic acids2. As a result, when taken up by recipient cells during the transport process, exosomes have the ability to modulate gene expression and cellular functions in the target cells3. As a kind of natural information messenger, exosomes have been fully taken advantage of in tissue regeneration, immune regulation, and as a delivery carrier4. Through engineering techniques, specific ligands can be enriched on the surface of exosomes, enabling the induction or inhibition of signaling events in recipient cells or targeting specific cell types5. Chemotherapeutic agents can also be loaded into exosomes for cancer treatment6. Moreover, exosomes have the ability to cross the blood-brain barrier for therapeutic cargo delivery, making them highly promising for the treatment of brain disorders7. Compared to liposomes, exosomes exhibit enhanced cellular uptake and improved biocompatibility8. They are capable of efficiently entering other cells while demonstrating better tolerance and lower toxicity9. However, the traditional bolus injection of exosomes is prone to sequestration and rapid clearance by the liver, kidneys, and spleen in the bloodstream10. Moreover, exosomes have poor stability in vitro and are susceptible to storage conditions, which restrict their clinical applications11.
Microneedles, an array of micrometric-sized needle tips, have the capability to penetrate physiological barriers for the delivery of small molecule drugs12, proteins13, nucleic acids14, and nanomedicines15. Microneedles are precisely engineered to target lesions on the skin surface, and their dispersed tips ensure uniform drug distribution at the targeted site, thus amplifying their therapeutic impact16. The design and material composition of microneedles facilitate the dry storage of bioactive substances such as proteins and nucleic acids, enhancing their stability17. Traditional injection methods have a relatively short duration of action and can cause pain, inducing fear in patients18. The micrometer-sized length of microneedle minimizes tissue trauma and prevents nerve stimulation, thereby eliminating pain and improving patient compliance19. Additionally, the user-friendly nature of microneedles allows patients to self-administer the treatment without the need for specialized personnel16. In addition to the skin, microneedles can also be used in tissues such as the eyes20, oral mucosa21, heart22, and blood vessels23. The application of microneedles for the clinical delivery of exosomes provides a promising and prospective strategy.
Hence, we introduce an exosome-loaded microneedle (exo@MN) patch and disclose its fabrication method. The microneedle patches were fabricated using a two-step casting method, along with centrifugation and vacuum drying, which promotes the aggregation of exosomes at the microneedle tips, thereby enhancing delivery efficiency. Both the needle tips and backing were constructed using materials that exhibit excellent biocompatibility and water solubility. Trehalose and hyaluronic acid (HA) were incorporated as tip materials to provide protection for the exosomes, and polyvinylpyrrolidone (PVP) dissolved in absolute ethanol was chosen as the backing material. The morphology of the microneedle patch was characterized using microscopy and scanning electron microscope (SEM). The mechanical testing of the microneedle was assessed using a tensile meter to confirm their capability to penetrate the skin, and the release rate on pig skin was investigated to be 60 s. Furthermore, the morphology, size, and protein content of both fresh exosomes and exosomes in exo@MN were characterized using transmission electron microscope (TEM), nanoparticle tracking analysis (NTA), and western blotting (WB). The internalization of exosomes by dendritic cells (DCs) was characterized using confocal laser scanning microscope (CLSM), and the maturation of DCs was evaluated through flow cytometry. The morphological characterization and biological functions of the two types of exosomes are essentially consistent.
Protocol
This study does not require ethical clearance as the pig skin used for the experiments described in section 3 was purchased as edible pig ears from the market and not sourced from experimental animals.
1. Isolation of exosomes
- Cell culture
- Cultivate mouse ovarian epithelial cancer cells ID8 in Dulbecco's Modified Eagle Medium (DMEM) culture medium containing 10% fetal bovine serum and 1% penicillin-streptomycin solution (100×) (see Table of Materials) in Petri dishes with a diameter of 15 cm.
- Incubate ID8 cells in a CO2 incubator (see Table of Materials) at 37 °C and 5% CO2 until the cell density reaches 90%.
- Isolation of exosomes
- Remove the culture medium from the Petri dishes and wash twice with Dulbecco's phosphate-buffered saline (DPBS). Add 20 mL of DMEM without fetal bovine serum (see Table of Materials) and penicillin-streptomycin solution (see Table of Materials). Continue the incubation at 37 °C and 5% CO2 for 48 h.
- Collect the cell culture supernatant from 20 Petri dishes of 15 cm diameter. Centrifuge at 300 x g for 10 min at 4 °C and collect the supernatant for removing free cells.
- Centrifuge at 2000 x g for 10 min 4 °C, and collect the supernatant for removing cellular debris.
- Connect the 0.22 µm disposable vacuum filtration system (see Table of Materials) to the circulating water vacuum pump (see Table of Materials). Create a vacuum to allow the supernatant to pass through the 0.22 µm polyether sulfone membrane and enter the lower chamber of the filtration system, effectively removing vesicles or impurities larger than 0.22 µm from the supernatant.
- To connect the vacuum pump, turn on the pump switch and then connect it to the air inlet of the vacuum filtration system. When closing, disconnect the connection with the pump first and then turn off the vacuum pump. This procedure helps to prevent backflow of the vacuum pump and contamination of the culture fluid.
- Add 15 mL of supernatant to the inner tube of the 100 kDa ultrafiltration tube (see Table of Materials). Centrifuge at 3500 x g for 10 min at 4 °C, and remove the liquid from the outer tube.
NOTE: Retain particles larger than 100 kDa on the inner tube membrane, while proteins smaller than 100 kDa will be centrifuged to the outer tube along with the solution. The exosomes were larger than 100 kDa and were aggregated on the inner tube membrane. - Repeat the above steps until the total volume of the liquid is within 5 mL. Collect the liquid from the inner tube and add 1 mL of DPBS. Use a pipette to repeatedly pipette in the inner tube, ensuring the resuspension of the attached exosomes in DPBS, and then collect them.
- Centrifuge at 10000 x g for 60 min at 4 °C, and transfer the supernatant to a 6 mL quick-snap centrifuge tube (see Table of Materials). Seal the tube with a heat sealer.
- Centrifuge at 100,000 x g for 2 h 4 °C using an ultracentrifuge (see Table of Materials). Cut open the quick-snap centrifuge tube, remove the supernatant, and resuspend the pellet in 100 µL of DPBS. This is the exosome solution.
NOTE: During the process of resuspending the pellet, it is necessary to pipette and agitate the tube wall at least 200 times using a pipette.
2. Fabrication of exo@MN
- Preparation of master mold
- Construct a microneedle model with a 10 x 10 array using CAD software, featuring conical-shaped tips with the following parameters: a height of 1200 µm, a base diameter of 400 µm, and a pitch (distance between adjacent microneedles) of 900 µm.
- Use HTL resin as the material and print the master mold of the microneedle using a 3D printer (see Table of Materials).
- Immerse the master mold in absolute ethanol for 1 h to remove any resin adhering to the surface.
- Expose the mold to ultraviolet light for 5 min to cure it.
- Immerse the mold once again in absolute ethanol for 1 h, followed by drying it at 60 °C in a drying oven for 1 h. The master mold is ready.
- Preparation of production mold
- Mix A and B components of polydimethylsiloxane (PDMS, see Table of Materials) in a ratio of 10:1. Stir well and pour the mixture into a master mold.
- Place the PDMS mold under a pressure of 1 psi for 15 min to remove air bubbles from the PDMS mixture.
- Cure the PDMS mold at 60 °C for 2 h and then demold to obtain the production mold of the microneedle.
- Clean the production mold with ultrapure water before use.
- Preparation of solution
- Quantify the protein content of the exosome solution using a BCA assay kit (see Table of Materials). Add an appropriate amount of DPBS to achieve a concentration of 10 µg/µL for the exosome solution.
- Prepare a solution of 200 mg/mL trehalose (see Table of Materials) using DPBS as the solvent. Stir for 20 min to ensure full dissolution.
- Prepare a solution of 200 mg/mL hyaluronic acid (HA, see Table of Materials) using DPBS as the solvent. Stir overnight to ensure full dissolution.
- Prepare a solution of 150 mg/mL polyvinylpyrrolidone (PVP, see Table of Materials) using absolute ethanol as the solvent. Stir overnight to ensure full dissolution.
- Mix the exosome solution (10 µg/µL) and trehalose solution (200 mg/mL) in a 1:1 volume ratio. Then, add an equal volume of HA solution (200 mg/mL). Mix thoroughly to obtain the tip solution.
- Fabrication of exo@MN
- Process the surface of the PDMS mold using a plasma cleaner (see Table of Materials) for 30 s at a low grade to enhance its hydrophilicity.
- Add 40 µL of the tip solution to the PDMS mold. Centrifuge at room temperature (RT) and 3000 x g for 3 min to ensure the liquid fills the needle layer of the mold.
- Remove excess liquid in the mold and dry at RT in a vacuum drying oven (see Table of Materials) for 1 day.
- Add 200 µL of PVP solution to the PDMS mold. Centrifuge 3000 x g for 3 min at RT to ensure the liquid fills the backing layer of the mold.
- Dry the mold at RT in a drying oven for 1 day, then demold to obtain the exo@MN patch.
NOTE: Keep the exo@MN patch stored in a drying oven until ready for use.
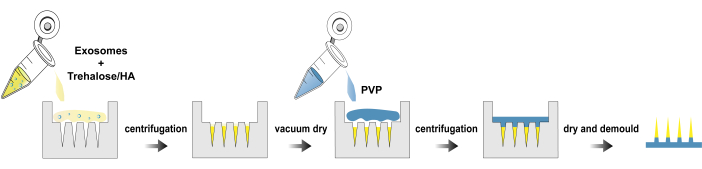
Figure 1: Fabrication process of exo@MN patches. Please click here to view a larger version of this figure.
3. Characterization of exo@MN patches
- Observation of morphology
- Paste the backing of the patch onto a 45° inclined surface and position it under a stereo microscope (see Table of Materials).
- Turn on the illuminator and capture the morphology of the patch using a 4x objective.
- Scanning electron microscopy (SEM)
- Attach the patch to the sample stage using conductive adhesive and treat it with an auto fine coater (see Table of Materials) for 30 s.
- Capture images of the exo@MN patch using SEM (see Table of Materials) with an accelerating voltage of 1 kV.
- Mechanical testing
- Cut out a 3 x 3 array from the patch and place it with the tips facing upwards on the rigid platform of a tensile meter (see Table of Materials). Adjust the height of the probe to bring it close to the needle tips without touching them.
- Set the parameters to stop when the pressure reaches 20 N automatically. Compress the needle tips vertically at a 0.5 mm/min speed and record the load versus displacement profile.
- Dissolution
- Purchase fresh pig ears from the market and cut them into pieces (5 cm x 5 cm x 0.3 cm). Spread the pig skin on a table and use paper to dry the surface moisture.
- Prepare four dry microneedle patches with the needle tips facing down on the pig skin, spacing each patch slightly apart. Using the thumb and index fingers, vertically press down on each microneedle patch. Remove one patch every 15 s.
- Immediately place the removed patch in a drying oven and dry for 5 min. Cut off a column of microneedle tips, place them horizontally under a microscope (see Table of Materials), and use a 4x objective lens to observe the dissolution of the tips.
4. Characterization of exosomes in exo@MN patch
NOTE: The microneedle tips of exo@MN are dissolved in 100 µL of DPBS solution to perform the following characterization on the released exosomes.
- Transmission electron microscopy (TEM)
- Treat the copper mesh (see Table of Materials) with a hydrophilic treatment using an ion cleaner (see Table of Materials) for 5 min.
- Dispense 10 µL of the sample onto the carbon side of the copper mesh. Let it stand for 1 min, then remove the liquid by blotting it with filter paper from the edges.
- Add 10 µL of 3% uranyl acetate (see Table of Materials) to the sample. Let it stand for 10 s, then remove the liquid by blotting with filter paper from the edges.
- Add 10 µL of 3% uranyl acetate to the sample. Let it stand for 1 min, then remove the liquid by blotting it with filter paper from the edges and air dry at RT.
- Capture images of the exosomes using TEM (see Table of Materials) with an accelerating voltage of 80 kV.
- Nanoparticle tracking analysis (NTA)
- Dilute the exosome solution with DPBS to achieve a particle concentration of 1 x 107 particles/mL. Inject slowly 1 mL of the exosome solution into the sample chamber of the NTA using 1 mL syringe (see Table of Materials).
- Set the instrument's standard operating procedure (SOP) type to EV-488 to detect the size distribution of the exosomes.
- Western blotting (WB)
- Prepare the lysis buffer by mixing radioimmunoprecipitation assay (RIPA) lysis buffer: phenylmethanesulfonyl fluoride (see Table of Materials) in a ratio of 100:1. Lyse the exosomes on ice for 20 min, vortexing every 10 min.
- Centrifuge the solution at 12,000 x g for 10 min at 4 °C. Collect the supernatant to measure the protein concentration.
- Add 5x SDS-PAGE loading buffer (see Table of Materials) to the supernatant in a volume ratio of 1 : 4 and mix well. Heat at 100 °C for 10 min to denature the proteins.
- Add 5 µL of a 180 kDa pre-stained protein marker (see Table of Materials) and 10 µg of denatured sample to a 4%-20% precast gel (see Table of Materials). Run the electrophoresis system (see Table of Materials) at 60 V for 10 min and then at 140 V for 50 min.
- Transfer the proteins from the gel to a PVDF membrane (see Table of Materials) pre-activated with methanol. Perform the electrophoresis system at 290 mA for 90 min on ice.
- Incubate the PVDF membrane in 5% skim milk solution for 1 h. Wash three times with tris buffered saline with tween (TBST, see Table of Materials) for 5 min each.
- Cut out the target bands based on the position of the protein. Incubate the bands with anti-mouse CD63 antibody and anti-mouse Alix antibody (see Table of Materials) diluted to the appropriate concentration according to the manufacturer's instructions, and incubate overnight at 4 °C.
- Wash the bands three times with TBST. Incubate with HRP-conjugated anti-rabbit IgG (see Table of Materials) at RT for 1 h. Then, wash three times with TBST again.
- Apply a super ECL detection reagent (see Table of Materials) to cover the surface of the bands, and immediately expose and capture the bands using a gel imager (see Table of Materials).
- Confocal laser scanning microscopy (CLSM)
- Cultivate 5 x 105 DCs in a confocal dish using 2 mL of Roswell Park Memorial Institute 1640 (RPMI 1640) medium (see Table of Materials). Add exosomes or exo@MN, and incubate at 37 °C for 24 h.
- Add a drop of Hoechst 33342 to each confocal dish and incubate in the dark at 37 °C for 20 min.
- Perform imaging with a CLSM (see Table of Materials) using a 60x objective lens. Apply a drop of immersion oil (see Table of Materials) on the lens and place the confocal dish on the stage. Adjust the x/y/z axes to locate the appropriate focal plane of the cells.
- Initiate imaging with DAPI/TRITC/TD channels, select a resolution of 1024 px, and set the fast mode to 1/8 s.
- Flow cytometry
- Cultivate DCs in a 6-well plate, each well containing 5 x 105 cells and 2 mL of 1640 medium. Add equal volumes of solution of DPBS, exosomes, microneedles without exosomes, and exo@MN, respectively, and incubate at 37 °C for 24 h.
- Transfer the medium from each well into centrifugal tubes. Centrifuge at 300 x g for 3 min at 4 °C, and remove the supernatant.
- Add 0.5 µL of FITC-CD11c antibody, 2.5 µL of APC-CD80 antibody, and 0.5 µL of Pacific Blue I-A/I-E antibody (see Table of Materials) to 100 µL of 5% BSA solution (see Table of Materials). Mix well, resuspend the cells, and incubate on a shaker in ice and in the dark for 20 min.
- Add 1 mL of DPBS and centrifuge at 300 x g for 3 min at 4 °C, then remove the supernatant. Repeat the wash twice to remove unbound antibodies, and then proceed to analyze the corresponding fluorescent channels via flow cytometry (see Table of Materials).
- First, in the FSC-A/SSC-A scatter plot, gate the main cell population as P1. In the SSC-A/SSC-H scatter plot of the P1 cell population, gate a square P2 region along the diagonal to remove aggregated cells.
- Perform fluorescence analysis on the P2 cell population using the FITC, APC, and Pacific Blue channels. Set the stop condition to collect 10,000 cells in the P2 gate.
5. Statistical analysis
- Express quantitative data as means ± standard deviations (SD). Assess statistical significance using t-test analysis using an appropriate data analysis software application. Consider p-values less than 0.05 to indicate statistical significance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Here, we present a protocol for the isolation of exosomes, fabrication and characterization of exo@MN patch. Figure 1 illustrates the process flowchart for the fabrication of exo@MN patch. The exosomes were mixed with trehalose and HA, and the mixture was then added to the microneedle mold and centrifuged. This process facilitated the aggregation of exosomes at the needle tips, promoting rapid release. After drying, PVP solution was added and centrifuged to fill the mold completely. Upon com...
Discussion
Currently, the main methods for isolating exosomes include ultracentrifugation, density-gradient centrifugation, ultrafiltration, precipitation, immunoaffinity magnetic beads, and microfluidics24. Due to the limited loading capacity of microneedles caused by their small needle tip space, it is necessary to increase the concentration of exosomes to load more. Therefore, we chose ultrafiltration to concentrate the cell culture supernatant and then used ultracentrifugation to isolate the exosomes. Th...
Disclosures
H. C., F.L.Q, and S.J.M are inventors in a patent application that has been filed based on the data in this manuscript. H.C. is the scientific founder of Medcraft Biotech. Inc.
Acknowledgements
F.L.Q. appreciates the support by supported by the Pioneer R&D Program of Zhejiang (2022C03031), the National Key Research and Development Program of China (2021YFA0910103), the National Natural Science Foundation of China (22274141, 22074080), the Natural Science Foundation of Shandong Province (ZR2022ZD28) and the Taishan Scholar Program of Shandong Province (tsqn201909106). H.C. acknowledges the financial support from the National Natural Science Foundation of China (82202329). The authors acknowledge the use of instruments at the Shared Instrumentation Core Facility at the Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences.
Materials
| Name | Company | Catalog Number | Comments |
| 100x penicillin-streptomycin solutions | Jrunbio Scientific | MA0110 | Cell culture |
| 180 kDa pre-stained protein marker | Thermo | 26616 | Western blotting |
| 3% Uranyl acetate | Henan Ruixin Experimental Supplies | GZ02625 | Morphological characterization of exosomes |
| 3D printer | BMF technology | nanoArch S130 | Mold preparation |
| 4%–20% precast gel | Genscript | ExpressPlus PAGE GEL | Western blotting |
| 5× SDS-PAGE loading buffer | Titan | 04048254 | Western blotting |
| Anti-mouse Alix antibody | Biolegend | 12422-1-AP | Western blotting |
| Anti-mouse CD63 antibody | Biolegend | ab217345 | Western blotting |
| APC anti-mouse CD80 antibody | Biolegend | 104713 | Antibody |
| Auto fine coater | ZIZHU | JBA5-100 | Morphological characterization of microneedle |
| BCA assay kit | Beyotime | P0012 | Protein concentration assay |
| Centrifuge | Thermo Fisher | Muitifuge X1R pro | Cell centrifuge |
| Circulating water vacuum pump | Yuhua Instrument | SHZ-D(III) | Filtration |
| CO2 incubator | Eppendorf | CellXpert C170 | Cell culture |
| Confocal laser scanning microscope | Nikon | A1HD25 | Fluorescence imaging |
| Copper mesh | Beijing Zhongjingkeyi Technology | JF-ZJKY/300 | Morphological characterization of exosomes |
| D- (+) -Trehalose dihydrate | Aladdin | 5138-23-4 | Fabrication of microneedle |
| Dulbecco’s modified Eagle’s medium | Meilunbio | MA0212 | Cell culture |
| Dulbecco’s phosphate-buffered saline | Meilunbio | MA0010 | Cell culture |
| Electrophoresis system | Bio-rad | PowerPac-basic | Western blotting |
| Fetal bovine serum | Jrunbio Scientific | JR100 | Cell culture |
| FITC anti-mouse CD11c antibody | Biolegend | 117305 | Antibody |
| Flow cytometry | BD | LSR Fortessa | Fluorescence detection |
| Gel imager | Cytiva | Amersham ImageQuant 800 | Western blotting |
| HRP-conjugated anti-rabbit IgG | CST | 7074S | Western blotting |
| HTL resin | BMF technology | Mold preparation | |
| Hyaluronic acid (MW = 300 kDa) | Bloomage Biotechnology | 9004-61-9 | Fabrication of microneedle |
| Immersion oil | Nikon | MXA22168 | Fluorescence imaging |
| Ion cleaner | JEOL | EC-52000IC | Morphological characterization of exosomes |
| Microscope | Olympus | CKX53 | Observe the microneedle tip dissolving process |
| Mouse ovarian epithelial cancer cell ID8 | MeisenCTCC | CC90105 | Cell culture |
| Nanoparticle tracking analysis | Particle Metrix | ZetaView | Size analysis of exosomes |
| Pacific Blue anti-mouse I-A/I-E antibody | Biolegend | 107619 | Antibody |
| Phenylmethanesulfonyl fluoride | Beyotime | ST507 | Protease inhibitors |
| Plasma cleaner | Hefei Kejing Material Technology | PDC-36G | Fabrication of microneedle |
| Polydimethylsiloxane | Dow Corning | 9016-00-6 | Mold preparation |
| Polyvinylpyrrolidone (MW = 40 kDa) | Aladdin | 9003-39-8 | Fabrication of microneedle |
| Prism | GraphPad | Version 9 | Statistical analysis |
| PVDF membrane | Millipore | IPVH00010 | Western blotting |
| Quick-snap centrifuge | Beckman | 344619 | Exosomes extraction |
| RIPA lysis buffer | Applygen | C1053 | Lysis membrane |
| Roswell park memorial institute 1640 | Meilunbio | MA0548 | Cell culture |
| Scanning electron microscope | JEOL | JSM-IT800 | Morphological characterization of microneedle |
| Stereo microscope | Olympus | SZX16 | Characterization of morphology |
| Super ECL detection reagent | Applygen | P1030 | Western blotting |
| Tensile meter | Instron | 68SC-05 | Mechanical testing |
| Transmission electron microscope | JEOL | JEM-2100plus | Morphological characterization of exosomes |
| Tris buffered saline | Sangon Biotech | JF-A500027-0004 | Western blotting |
| Tween-20 | Beyotime | ST825 | Western blotting |
| Ultracentrifuge | Beckman | Optima XPN-100 | Exosomes extraction |
| Ultrafiltration tube | Millipore | UFC910096 | Exosomes concentration |
| Vacuum drying oven | Shanghai Yiheng Technology | DZF-6024 | Fabrication of microneedle |
| Vacuum filtration system | Biosharp | BS-500-XT | Filtration |
References
- Barile, L., Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 174, 63-78 (2017).
- Kalluri, R., Lebleu, V. S. The biology, function, and biomedical applications of exosomes. Science. 367 (640), eaau6977 (2020).
- Mathieu, M., Martin-Jaular, L., Lavieu, G., Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 21 (1), 9-17 (2019).
- Nair, A., et al. Hybrid nanoparticle system integrating tumor-derived exosomes and poly(amidoamine) dendrimers: Implications for an effective gene delivery platform. Chem Mater. 35 (8), 3138-3150 (2023).
- Lu, Z., et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 67 (4), 739-748 (2017).
- Oskouie, M. N., Aghili Moghaddam, N. S., Butler, A. E., Zamani, P., Sahebkar, A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J Cell Physiol. 234 (6), 8182-8191 (2019).
- Yuan, D., et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 142, 1-12 (2017).
- Liao, W., et al. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 86, 1-14 (2019).
- Zhu, X., et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from hek293t cells. J Extracell Vesicles. 6 (1), 1324730 (2017).
- Wen, S., et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a radiation injury bone marrow murine model. Int J Mol Sci. 20 (21), 5468-5482 (2019).
- Cheng, Y., Zeng, Q., Han, Q., Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 10 (4), 295-299 (2019).
- Jana, B. A., Shivhare, P., Srivastava, R. Gelatin-PVP dissolving microneedle-mediated therapy for controlled delivery of nifedipine for rapid antihypertension treatment. Hypertens Res. 47 (2), 427-434 (2024).
- Zheng, Y., et al. A rapidly dissolvable microneedle patch with tip-accumulated antigens for efficient transdermal vaccination. Macromol Biosci. 23 (12), e2300253 (2023).
- Huang, D., et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics. 8 (9), 2361-2376 (2018).
- Zhou, Z., et al. Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-pd1 immunotherapy via nanovaccine complexed microneedle. Nano Res. 13 (6), 1509-1518 (2020).
- Kim, Y. C., Park, J. H., Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 64 (14), 1547-1568 (2012).
- Bui, V. D., et al. Dissolving microneedles for long-term storage and transdermal delivery of extracellular vesicles. Biomaterials. 287, 121644 (2022).
- Nir, Y., Potasman, I., Sabo, E., Paz, A. Fear of injections in young adults: Prevalence and associations. Am J Trop Med Hyg. 68 (3), 341-344 (2003).
- Zhang, Y., Jiang, G., Yu, W., Liu, D., Xu, B. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater Sci Eng C Mater Biol Appl. 85, 18-26 (2018).
- Roy, G., Garg, P., Venuganti, V. V. K. Microneedle scleral patch for minimally invasive delivery of triamcinolone to the posterior segment of eye. Int J Pharm. 612, 121305 (2022).
- Creighton, R. L., Woodrow, K. A. Microneedle-mediated vaccine delivery to the oral mucosa. Adv Healthc Mater. 8 (4), 1801180 (2019).
- Hu, S., Zhu, D., Li, Z., Cheng, K. Detachable microneedle patches deliver mesenchymal stromal cell factor-loaded nanoparticles for cardiac repair. ACS Nano. 16 (10), 15935-15945 (2022).
- Yang, W., Zheng, H., Lv, W., Zhu, Y. Current status and prospect of immunotherapy for colorectal cancer. Int J Colorectal Dis. 38 (1), 266-276 (2023).
- Zhang, M., et al. Methods and technologies for exosome isolation and characterization. Small Methods. 2 (9), 1800021 (2018).
- Bosch, S., et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 6, 36162 (2016).
- Bhattacharyya, M., Jariyal, H., Srivastava, A. Hyaluronic acid: More than a carrier, having an overpowering extracellular and intracellular impact on cancer. Carbohydr Polym. 317, 121081 (2023).
- Chang, H., et al. Cryomicroneedles for transdermal cell delivery. Nat Biomed Eng. 5 (9), 1008-1018 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved